
Volume 3, Issue 2
Insights in Analytical Electrochemistry
ISSN: 2470-9867
Analytical Chemistry-Formulation 2017
August 28-30, 2017
Page 24
8
th
Annual Congress on
&
14
th
International Conference and Exhibition on
August 28-30, 2017 Brussels, Belgium
Analytical and Bioanalytical Techniques
Pharmaceutical Formulations
Optimizing early phase development of amorphous solid dispersion formulation thorough application
of modeling tools
Samuel Kyeremateng
AbbVie Deutschland GmbH & Co. KG, Germany
Statement of the Problem:
Amorphous Solid Dispersion (ASD) is an established formulation technique for improving the
bioavailability of poorly water-soluble Active Pharmaceutical Ingredients (APIs) by increasing solubility, wettability and
dissolution rate. Successful manufacturing of ASD formulation by Hot Melt Extrusion (HME) requires selection of e.g. the
right API load, excipients, and processing temperature. API load is also crucial in determining important quality attributes of
the drug product such as long term physical stability to ensure consistent product performance during its self-life. Identifying
the possible maximum drug load limit and excipients for HME feasibility and risk assessment, and long-term physical stability
of the manufactured ASD can be quite challenging whereby several extrusion trials are required in addition to prolonged
stability studies. Exploring the optimal design space during early phase of formulation development by this approach requires
significant amount of resources including API which may be limitedly available during this phase.
Methodology&TheoreticalOrientation:
As anAPI-sparing approach, novel empiricalmodel and the rigorous thermodynamic
Perturbed Chain Statistically Associating Fluid Theory (PC-SAFT) were applied to model ASD phase diagram of several
formulations to effectively and quickly explore the design space to optimize formulation development. These were followed up
with HME manufacturing and long-term stability studies (up to 18 months) of the formulations under ICH conditions to verify
the model-predicted results. Several APIs and polymeric excipients including Soluplus, Copovidone, PVP, and HPMCAS were
used in the studies.
Findings:
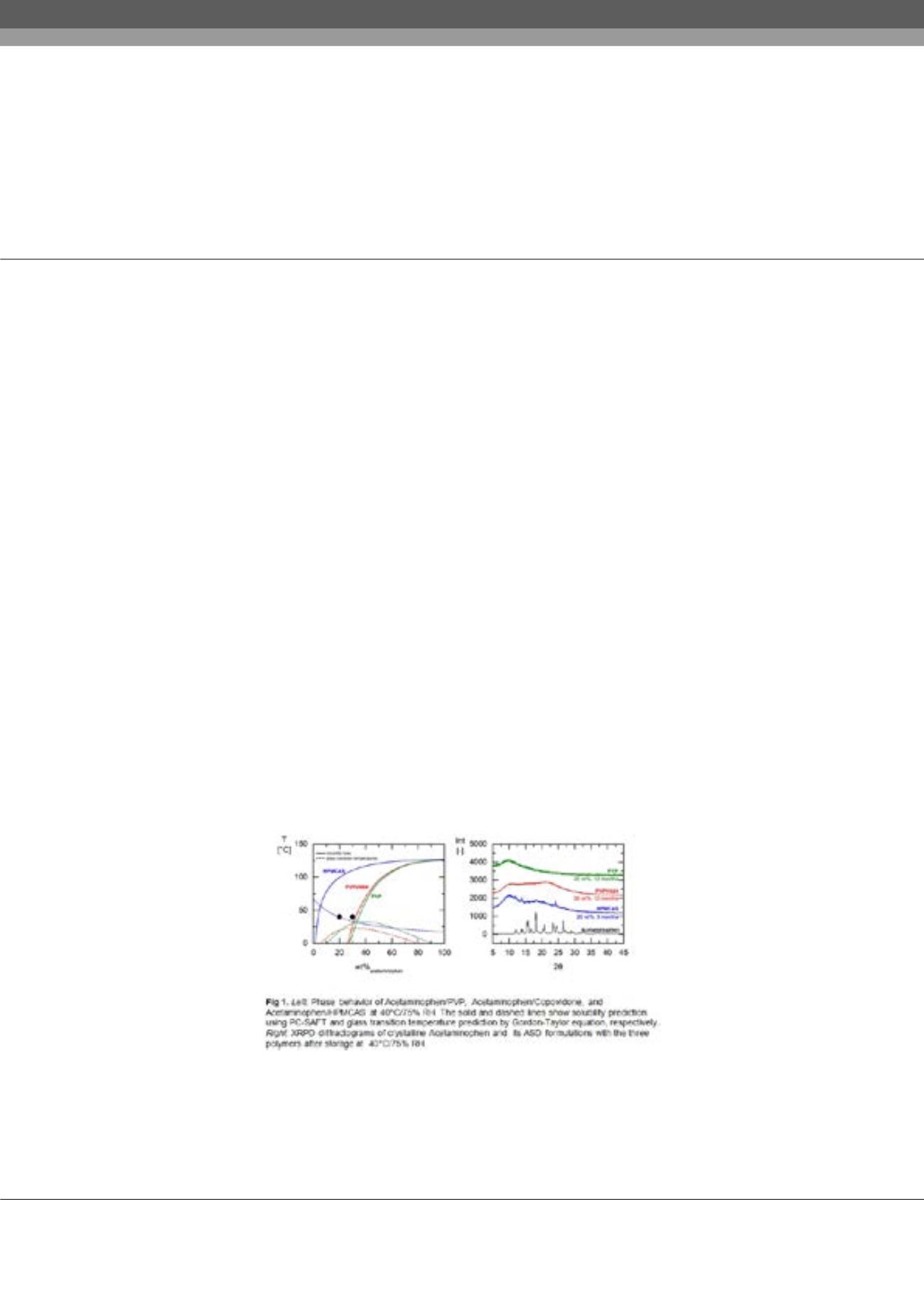
The modeling tools were found to be very suitable in estimating extrusion temperature required for generating
crystal-free ASD formulations as well as predicting their physical stability under different storage conditions, i.e., temperature
and relative humidity.
Conclusion & Significance:
Recent advances in predictive ASD phase diagram modeling proved to be reliable tools for
excipient selection, HME temperature prediction, and designing ASD formulations for maximum drug load and physical
stability. Applying these tools enables successful ASD formulation optimization using less resources and materials.
Biography
Samuel Kyeremateng is a Senior Scientist in the Global Pharmaceutical Sciences Division at AbbVie Deutschland in Ludwigshafen. His research activities focus
on scientific advances in the understanding of amorphous molecular solids, and development and application of models in predicting with confidence the preferred
composition, manufacturing process, and stability of amorphous solid dispersion formulations. His current responsibilities at AbbVie Deutschland include leading
the Material Science Group that supports formulation development, and mentoring Doctorate research students and other scientists within the company. He
received his Doctorate in Polymer Science from Martin-Luther-Universität in Germany.
samuel.kyeremateng@abbvie.comSamuel Kyeremateng, Insights in Analytical Electrochemistry, 3:2
DOI: 10.21767/2470-9867-C1-002
















