
Medchem & Toxicology 2018
Page 84
Journal of Organic & Inorganic Chemistry
ISSN: 2472-1123
A n n u a l C o n g r e s s o n
Medicinal Chemistry,
Pharmacology and toxicology
J u l y 3 0 - 3 1 , 2 0 1 8
Am s t e r d a m , N e t h e r l a n d s
C
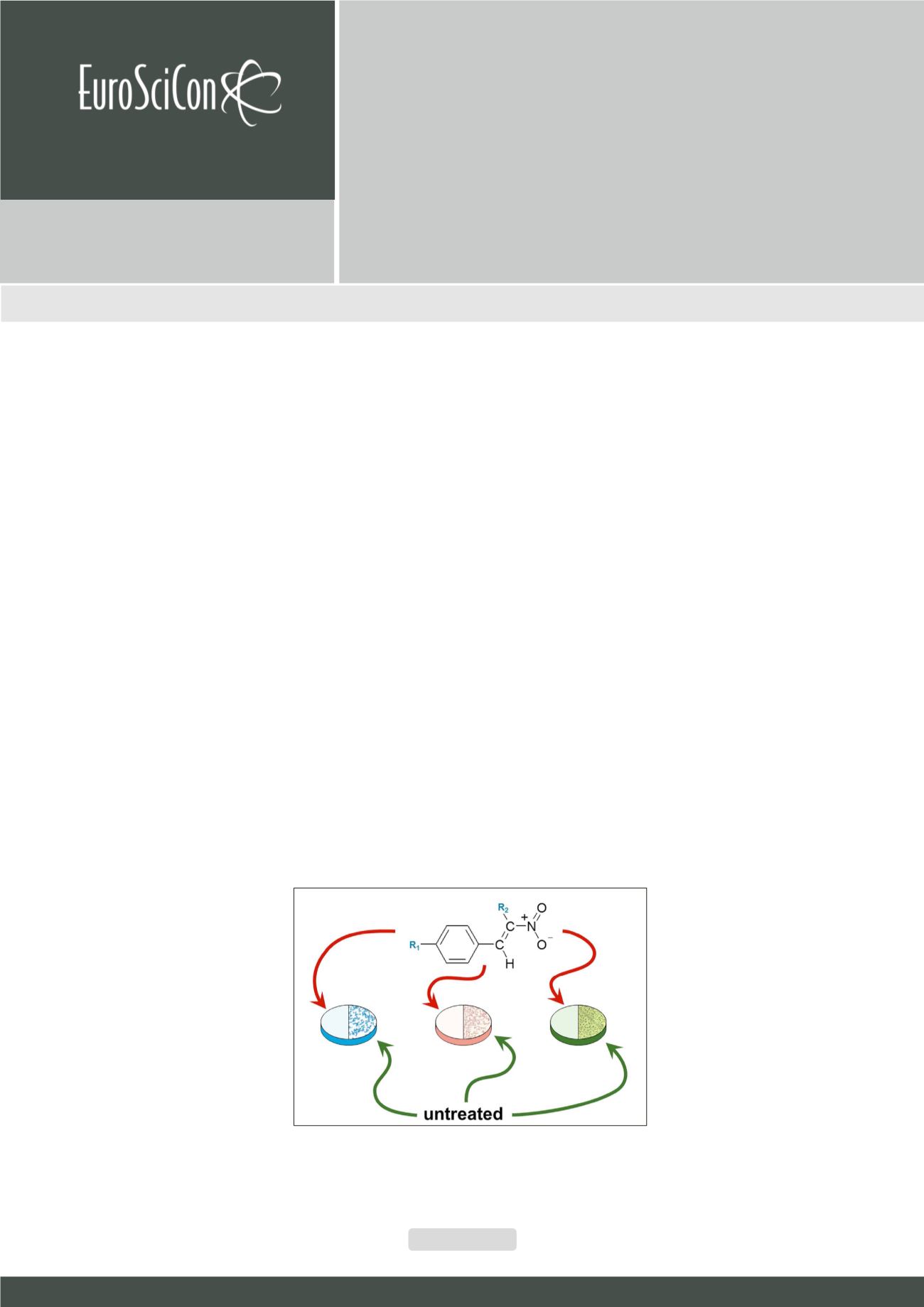
onjugated nitroalkenes are valuable precursors in organic synthesis they have significant affinity to nucleophilic reagents
as diazocompounds, nitrones, ylides, carbodienes, vinyl ethers etc., additionally; nitro group may be easily converted to
many other functional groups. Subsequently it was known, that nitro group conjugated with sp
2
carbon atom stimulate many
forms of biological activity. Considering these facts we decided to check a series of (E)-2-aryl-1-cyano-1-nitroethenes as
potential antibacterial and antifungal agents but without genotoxic properties. A homogenous series of β-EWG functionalyzed
β-nitrostyrenes were synthesized and characterized by IR, UV,
1
H-NMR and
13
C-NMR spectra. Obtained compounds were screened
in vitro
against a panel of reference strains of bacteria and fungi and their cytotoxicity towards cultured human HepG2 and HaCaT
cells was established. Antimicrobial results indicated that four of synthesized compounds exhibited significant antimicrobial
activity against all tested reference bacteria and fungi belonging to yeasts with a specific and strong activity towards
B. subtilis
ATCC 6633. The details of our studies, which describe the synthesis, activity, and proposition for the mechanism of an action of
studied compounds, will be presented
.
Oleh.Demchuk@UMCS.Lublin.plNew aspects of synthesis and microbiological
applications of
β
-EWG functionalized
β
-nitrostyrene
Oleg M Demchuk
1
, Anna Boguszewska-Czubara
2
, Karolina Kula
3
,
Artur Wnorowski
2
, Anna Biernasiuk
2
, Łukasz Popiołek
2
, Dawid
Miodowski
3
and Radomir Jasinski
3
1
Maria Curie-Skłodowska University, Lublin, Poland
2
Medical University of Lublin, Lublin, Poland
3
Cracow University of Technology, Cracow, Poland
J Org Inorg Chem 2018, Volume 4
DOI: 10.21767/2472-1123-C3-009
Figure.
Synthesis of 4-AN and 4-AN-Ac
















