
Medchem & Toxicology 2018
Page 82
Journal of Organic & Inorganic Chemistry
ISSN: 2472-1123
A n n u a l C o n g r e s s o n
Medicinal Chemistry,
Pharmacology and toxicology
J u l y 3 0 - 3 1 , 2 0 1 8
Am s t e r d a m , N e t h e r l a n d s
I
nhumans, tengeneraof fungi, i.e.Aspergillus, Candida, Cryptococcus, Blastomyces, Coccidioides, Histoplasma, Paracoccidioides,
Penicillium, Pneumocystis, and Rhizopus have a high prevalence in infections, with more than 40% associated with Candida.
Antifungal resistance is an increasing threat for the effective treatment of invasive mycoses, making their therapy difficult,
expensive, or even impossible. Therefore, there is an urgent need for new compounds targeting different cellular processes,
including phosphorylation, to deal with Candida infections. In this study, we applied various assays to find new activities of
phenylcyanomethylenequinone oxime (4-AN) for potential anti-microbial applications. These assays determined (a) the
antimicrobial effect on growth/cell multiplication in bacterial and fungal cultures; (b) the effect on
in vitro
activity of CK2, i.e.
one of the most pleiotropic kinases, and the Rio1 kinase, which is crucial for ribosome maturation; (c) hemolytic activity towards
human erythrocytes; and (d) toxicity against the Caco-2 cancer cell line. We demonstrated, for the first time, the activity of 4-AN
against selected bacteria and against Candida. At 125-250 µg/ml of the minimum inhibitory concentration (MIC), the chemical
significantly affected the bacterial strains. Interestingly, the MIC ranging from 3.9 µg/ml to 7.8 µg/ml showed effectiveness in
inhibition of formation of hyphae and cell aggregation in Candida, which was demonstrated at the cytological level. Notably, 4-AN
was found to inhibit the CK2 and Rio1 kinases with different potency. However, at low concentrations, it did not exert any evident
toxic effects on human cells. The details of our studies, which describe the synthesis, activity, and proposition for the mechanism
of an action of studied compounds will be presented
.
Oleh.Demchuk@UMCS.Lublin.plThe arylcyanomethylenequinone inhibition of growth
and formation of hyphae in Candida albicans
Oleg M Demchuk
1
, Maciej Masłyk
2
, Konrad Kubiński
2
, Monika
Janeczko
2
, Hieronim Golczyk
2
, Anna Sierosławska
2
and Anna
Rymuszka
2
1
Maria Curie-Skłodowska University, Lublin, Poland,
2
The John Paul II Catholic University of Lublin, Lublin, Poland
J Org Inorg Chem 2018, Volume 4
DOI: 10.21767/2472-1123-C3-009
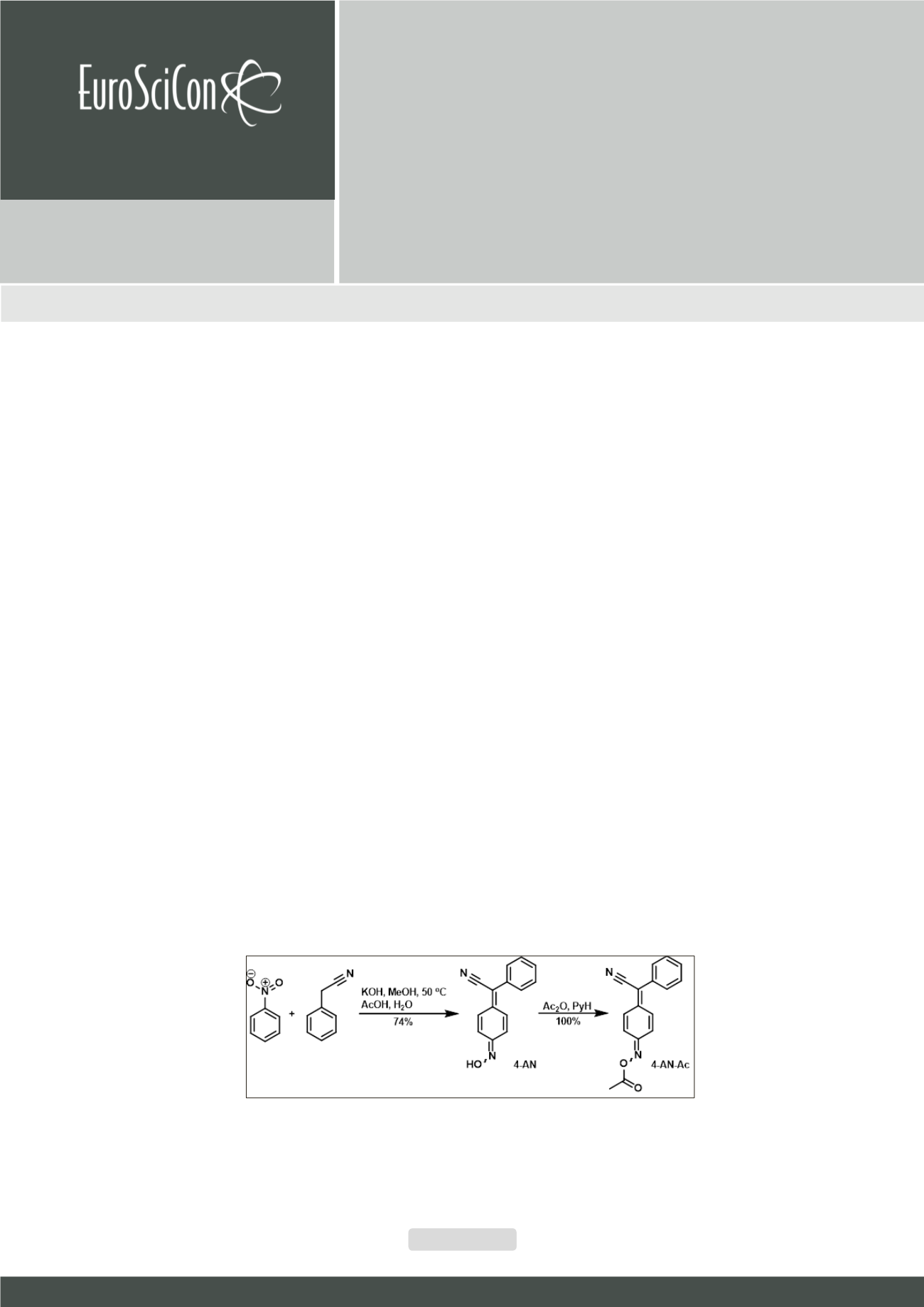
Figure 1
Synthesis of 4-AN and 4-AN-Ac
















