
E u r o S c i C o n C o n f e r e n c e o n
Chemistry
2018
Chemistry 2018
Journal of Organic & Inorganic Chemistry
ISSN 2472-1123
F e b r u a r y 1 9 - 2 0 , 2 0 1 8
P a r i s , F r a n c e
Page 22
S
ome cyanopolyynes (the formula of which is R-(C
≡
C)n-CN with R = H or
Me) have been detected in the interstellar medium (ISM) and on titan for
some of them. Our laboratory has been studying from several years these
compounds by synthesizing themand studying their chemical or photochemical
reactivity. However, despite the low number of atoms these compounds
possess, their synthesis in laboratory still remains a scientific challenge. Thus,
we have recently described a synthetic pathway for cyanopolyynes having two
conjugated C
≡
C triple bonds (n=2), but their superior counterparts (n=3) stay
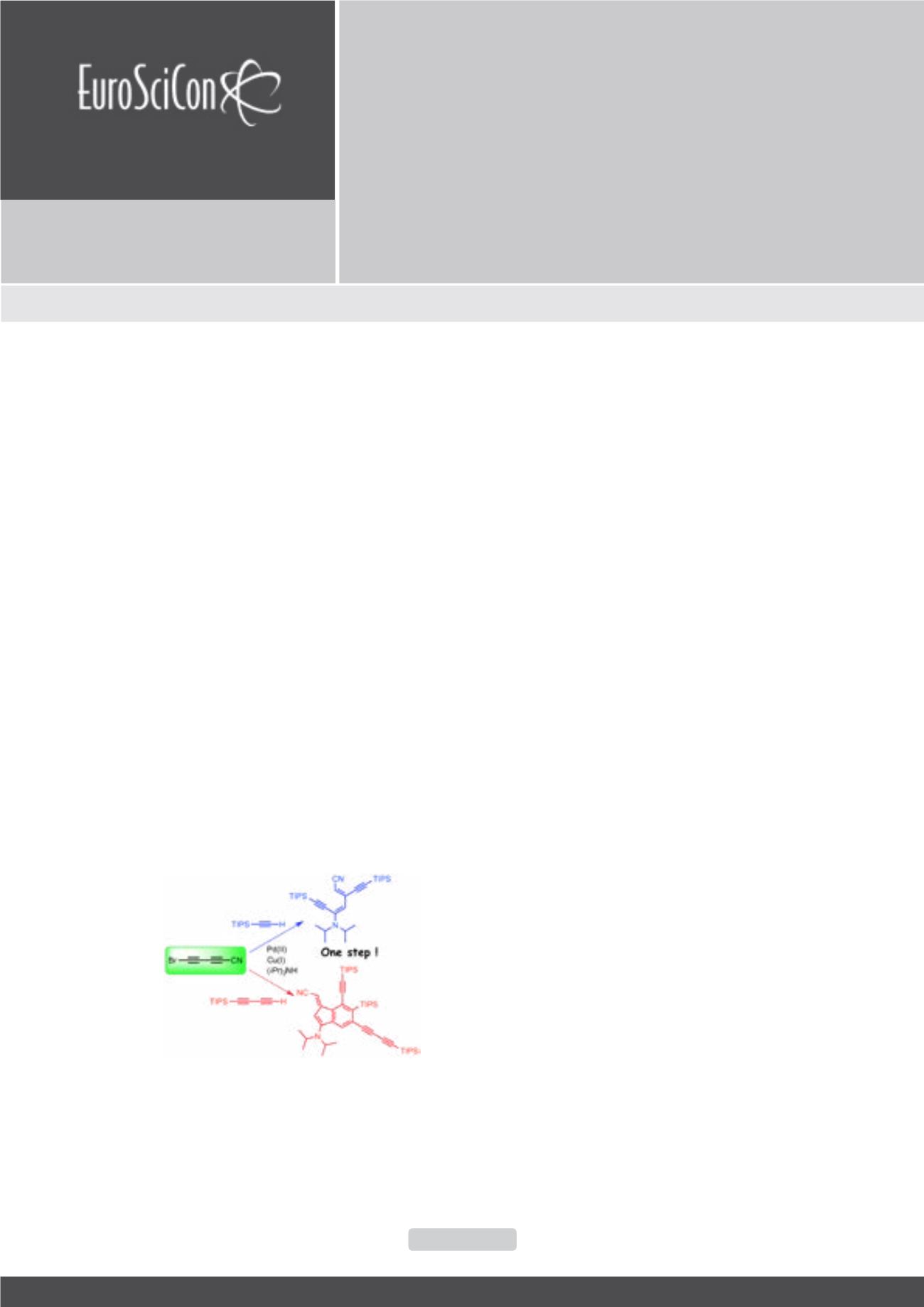
elusive so far. To solve this problem, we synthesized the bromocyanobutadiyne
(5-bromopenta-2,4- diynenitrile; Br-C
≡
C-C
≡
C-CN) and reacted it with
different terminal alkynes under Cadiot-Chodkiewicz conditions. Surprisingly,
the corresponding cyanopolyynes were not obtained but more complex
compounds, resulting from cascade reactions, were isolated. In particular, a
diene was obtained stereoselectively when using monoacetylenic reactants.
When using triisopropylsilylbutadiyne, a functionalized benzofulvene was
obtained. The characterization of these unexpected products is based on X-ray
crystallography, among other usual techniques. The mechanisms of formation
of these products, which were studied both experimentally and theoretically,
will be discussed.
Recent Publication
V Vuitton, R V Yelle and V G Anicich (2006) The
nitrogen chemistry of titan’s upper atmosphere
revealed. Astrophys. J. 647(2):L175-L178.
Y Trolez and J C Guillemin (2005) Synthesis
and characterization of 2,4-pentadiynenitrile
- A key compound in space science. Angew.
Chem. Int. Ed. 44:7224-7226.
N Kerisit, L Toupet, Y Trolez and J C Guillemin
(2013) Methylcyanobutadiyne: synthesis, X-ray
structure and photochemistry; towards an
explanation of its formation in the Interstellar
Medium. Chem. Eur. J. 19:17683-17686.
N Kerisit, C Rouxel, S Colombel Rouen, L
Toupet, J C Guillemin and Y Trolez (2016)
Synthesis, chemistry, and photochemistry of
methylcyanobutadiyne in the context of space
science. J. Org. Chem. 81:3560−3567.
N Kerisit, L Toupet, P Larini, L Perrin, J C
Guillemin and Y Trolez (2015) Straightforward
synthesis of 5-bromopenta-2,4-diynenitrile
and its reactivity towards terminal alkynes:
a direct access to diene and benzofulvene
scaffolds. Chem. Eur. J. 21:6042-6047
Biography
Yann Trolez has completed his PhD in 2010 from the Univer-
sity of Strasbourg under the guidance of Jean-Paul Collin and
Jean-Pierre Sauvage where he worked on rotaxane-based mo-
lecular machines. He then moved to ETH Zürich in François
Diederich group as a Postdoctoral Fellow where he worked on
alleno-acetylenic compounds bearing interesting optoelectron-
ic properties. In 2011, he was appointed as Assistant Professor
at the Ecole Nationale Supérieure de Chimie de Rennes where
he nowworks on organic interstellar chemistry and on new cya-
nated compounds having interesting optical properties. He has
published about 30 papers in peer-review international journals..
yann.trolez@ensc-rennes.frSynthesis and reactivity of bromocyanobutadiyne: from
interstellar chemistry to organic synthesis methodology
Yann Trolez
Ecole Nationale Supérieure de Chimie de Rennes - CNRS, France
Yann Trolez, J Org Inorg Chem 2018, Volume: 4
DOI: 10.21767/2472-1123-C1-002
Figure1: Reactivity of bromocyanobutadiyne with
triisopropylsilylacetylene and triisopropylsilylbutadiyne.
















