
E u r o S c i C o n C o n f e r e n c e o n
Chemistry
2018
Chemistry 2018
Journal of Organic & Inorganic Chemistry
ISSN 2472-1123
F e b r u a r y 1 9 - 2 0 , 2 0 1 8
P a r i s , F r a n c e
Page 54
T
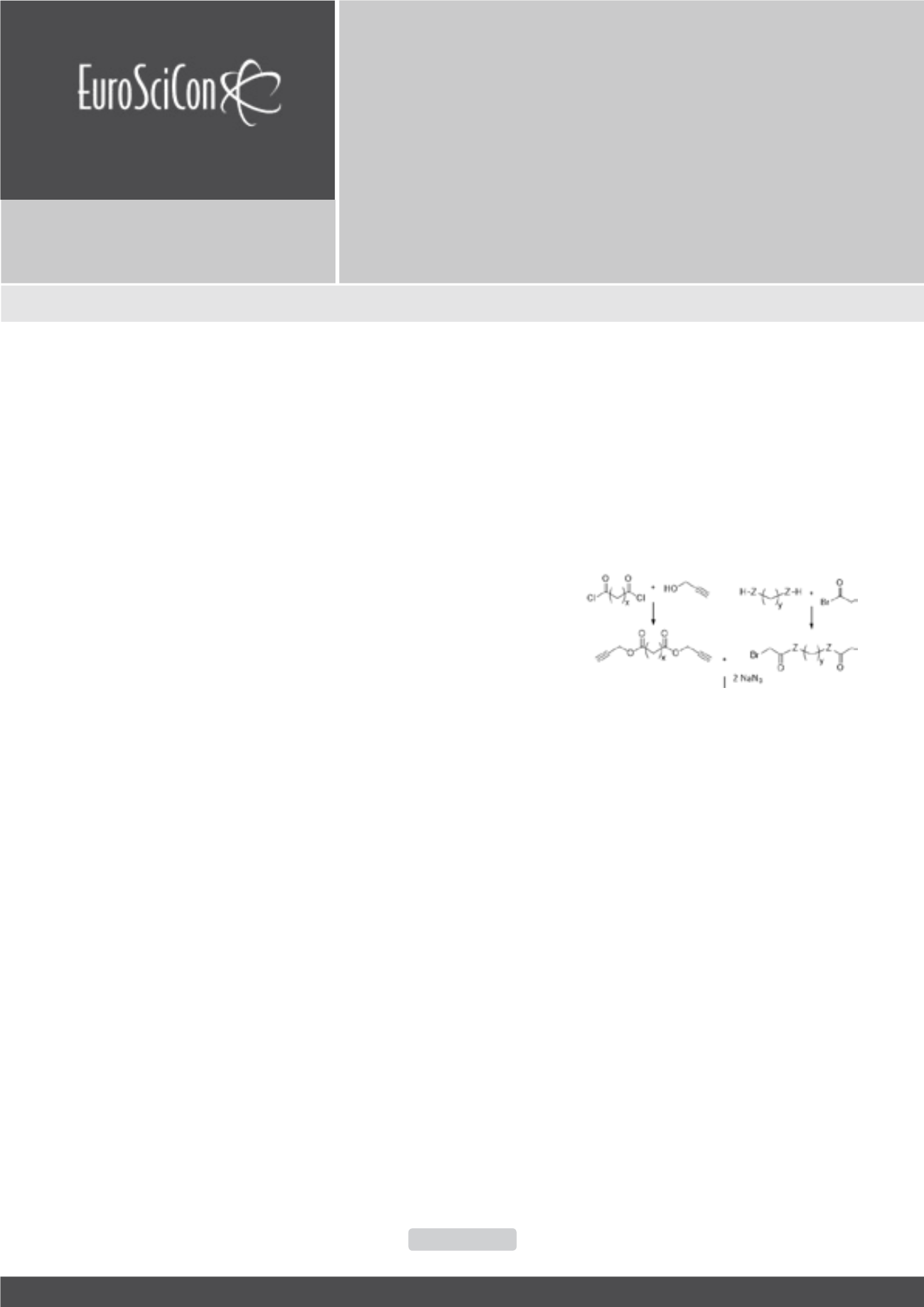
he use of click chemistry in polymer science is a quickly emerging
field of research. The copper (I) catalyzed azide–alkyne cycloaddition
(CuAAC) click reaction has already been exploited for the synthesis of end-
functionalized polymers, block copolymers, cyclic polymers, dendrimers,
cross-linked materials, etc. Surprisingly, there are only few papers on the
application of CuAAC click reaction in step-growth polymerization (SGP) as a
chain propagation reaction and to our best knowledge, there are no examples
of the synthesis of biodegradable polymers via CuAAC click chemistry. In
the present work we have carried out a systematic study for optimizing the
CuAAC click SGP reaction for the synthesis of clicking polyesters in terms
of solvent, catalyst, catalyst’s activator (ligand), monomers concent¬ration,
duration and temperature of various steps of one pot reaction. Comparing
the clicking polyester’s molecular weights and yields the best parameters for
the click SGP were found as: a solvent - N-Methyl-2-pyrrolidone, a catalyst
– CuI, a ligand – NEt3, a monomers concentration – 0.6 mol/L, duration of
bis-azide formation step – 3 h (at room temperature), duration of SGP – 15
h, temperature of the click SGP reaction – 0° C. The established optimal
conditions of the CuAAC-based SGP reaction was applied to the synthesis of
a series of high-molecular-weight (Mw up to 73,000 Da) 1,2,3-triazole cycles-
containing clicking polyesters and poly(ester amide)s (Scheme 1) which reveal
improved thermal properties compared to their regular analogues. The new
polymers are promising for practical applications in medicine, agriculture, and
food industry as biodegradable (bioresorbable) materials, as environmentally
friendly biomaterials, etc. Furthermore, one of the important advantages of the
developed CuAAC click SGP is the possibility of quaternization of 1,2,3-triazole
cycles of the resulting polymers which opens a way to cationic polymers –
both water soluble ones and cross-linked cationic hydrogels promising for
numerous biomedical applications.
Biography
Tengiz Kantaria has his expertise in the preparation and char-
acterization of nanoparticles on the basis of amino acid based
biodegradable poly(ester amide)s (MS thesis, 2015). Currently
as a PhD student he is engaged in the synthesis and characteri-
zation of new biodegradable polymers (polyesters, polyamides,
and poly(ester amide)s) via Cu(I) catalyzed alkyne–azide 1,3-cy-
cloaddition click reaction.
t.kantaria@agruni.edu.geClick chemistry-based step growth polymerization: a new
approach for the synthesis of novel clicking biodegradable
polymers
Tengiz Kantaria, Tem Kantaria, G Otinashvili, N Kupatadze, N
Zavradashvili, D Tugushi and R Katsarava
Agricultural University of Georgia, Georgia
Tengiz Kantaria, J Org Inorg Chem 2018, Volume: 4
DOI: 10.21767/2472-1123-C1-003
















