
Medchem & Toxicology 2018
Page 71
Journal of Organic & Inorganic Chemistry
ISSN: 2472-1123
A n n u a l C o n g r e s s o n
Medicinal Chemistry,
Pharmacology and toxicology
J u l y 3 0 - 3 1 , 2 0 1 8
Am s t e r d a m , N e t h e r l a n d s
I
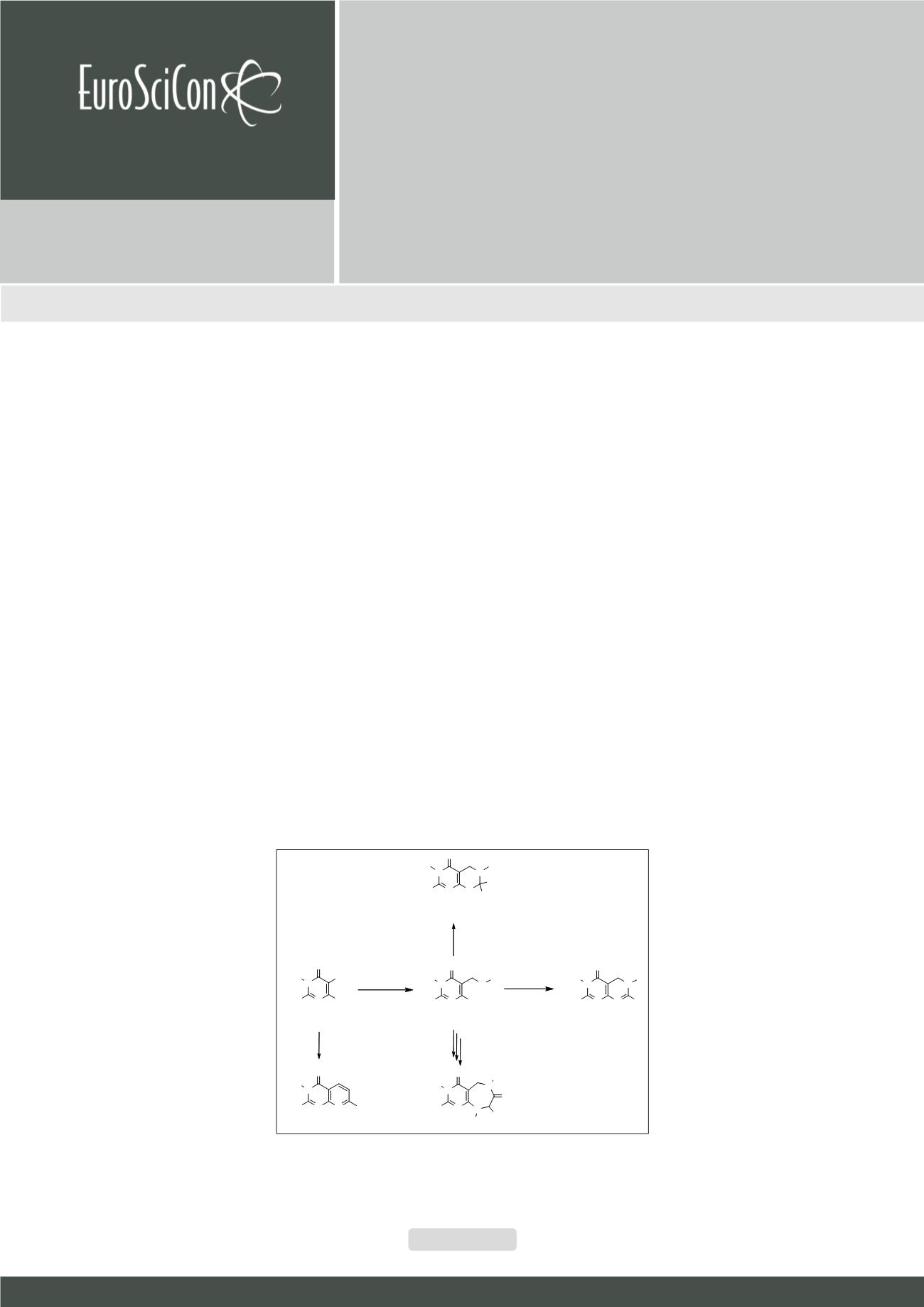
t’s well known the biological importance of pyrimidine nucleus in essential natural occurring products such nucleic acids; showing
the related compounds a great diversity of pharmaceutical properties, i.e. antibiotic, antitumoral or antifungal agents. Some
mimic pteridine derivatives have been prepared by different pathways using 4-Aminopyrimidine-5-carbaldehydes (I) as starting
materials. The related compounds are 5,6-dihydropyrimido[4,5-d] pyrmidines (III), 5,6,7,8-tetrahydropyrimido[4,5-d] pyrmidines
(IV) and pyrimido [4,5-e] diazepines (V) all obtained via 4-amino-(5-aminomethyl)pyrimidine intermediates (II). In addition, some
7-arylpyrido [2, 3-d] pyrimidines (VI) have been prepared by Friedländer type synthesis starting from the same carbaldehydes (II).
The dihydro derivatives (III) were prepared means of a final cyclocondensation carried out with orthoesters, catalysed by acid and
assisted by microwaves irradiation under solvent free conditions. The final cyclocondensation with carbonyl compounds forming
the tetrahydro derivatives (IV) was done under mild conditions, in which stereochemical induction was carried out on the building
of this skeleton, and stereochemistry assignments corroborated by theoretical calculations. Pyrimido [4,5-e] [1,4] diazepines (V)
were obtained by a two-step acylation/cyclization sequence from key intermediates 6-amino-5-(amino) methylpyrimidines (II)
have been carried out. The 7-arylpyrido [2, 3-d] pyrimidines derivatives (VI) have been synthesized by a Friedländer type reaction
with acetophenones under solvent-free conditions and in the presence of BF
3
-Et
2
O. All these methodologies are straightforward
and inspired in Diversity-Oriented Synthesis (DOS). The isolation of the desired products are simple and in good yields.
jdelatorre@nanomyp.comPyrimidin-5-carbaldehydes as intermediates in the
synthesis of non-common fused pyrimidinic systems
J M de la Torre
1
, J Cobo
2
and M Nogueras
2
1
NanoMyP® (Nanomateriales y Polímeros), Spain
2
University of Jaen, Spain
J Org Inorg Chem 2018, Volume 4
DOI: 10.21767/2472-1123-C3-009
Figure:
Diversity Oriented Synthesis of
non-common fused pyrimidinic systems
N
N
O
H
3
C
H
3
CX
NH
2
NH
2
R
1
CHO NaBH ( OAc )
3
I
II
N
N
O
H
3
C
H
3
CX
NH
2
N
H
R
1
N
N
O
H
3
C
H
3
CX
N
N
R
1
R
2
III
R
2
C ( OR '
)
3
MW
PTSA
( cat )
/ EtOH
N
N N
H
N
O
H
3
C
H
3
CX
R
1
R
2
R
3
IV
R
2
R
3
CO
N
N
O
H
3
C
H
3
CX
VI
N Ar
CH
3
COAr
Fusion
BF
3
-
Et
2
O
N
N
O
H
3
C
H
3
CX
V
N
N
R
1
R
3
O
R
2
















