
Volume 3, Issue 2
Insights in Analytical Electrochemistry
ISSN: 2470-9867
Analytical Chemistry-Formulation 2017
August 28-30, 2017
Page 47
8
th
Annual Congress on
&
14
th
International Conference and Exhibition on
August 28-30, 2017 Brussels, Belgium
Analytical and Bioanalytical Techniques
Pharmaceutical Formulations
Long-term stability of pharmaceutical formulations - prediction of recrystallization and amorphous-
amorphous phase separation
Christian Luebbert
and
Gabriele Sadowski
TU Dortmund University, Germany
N
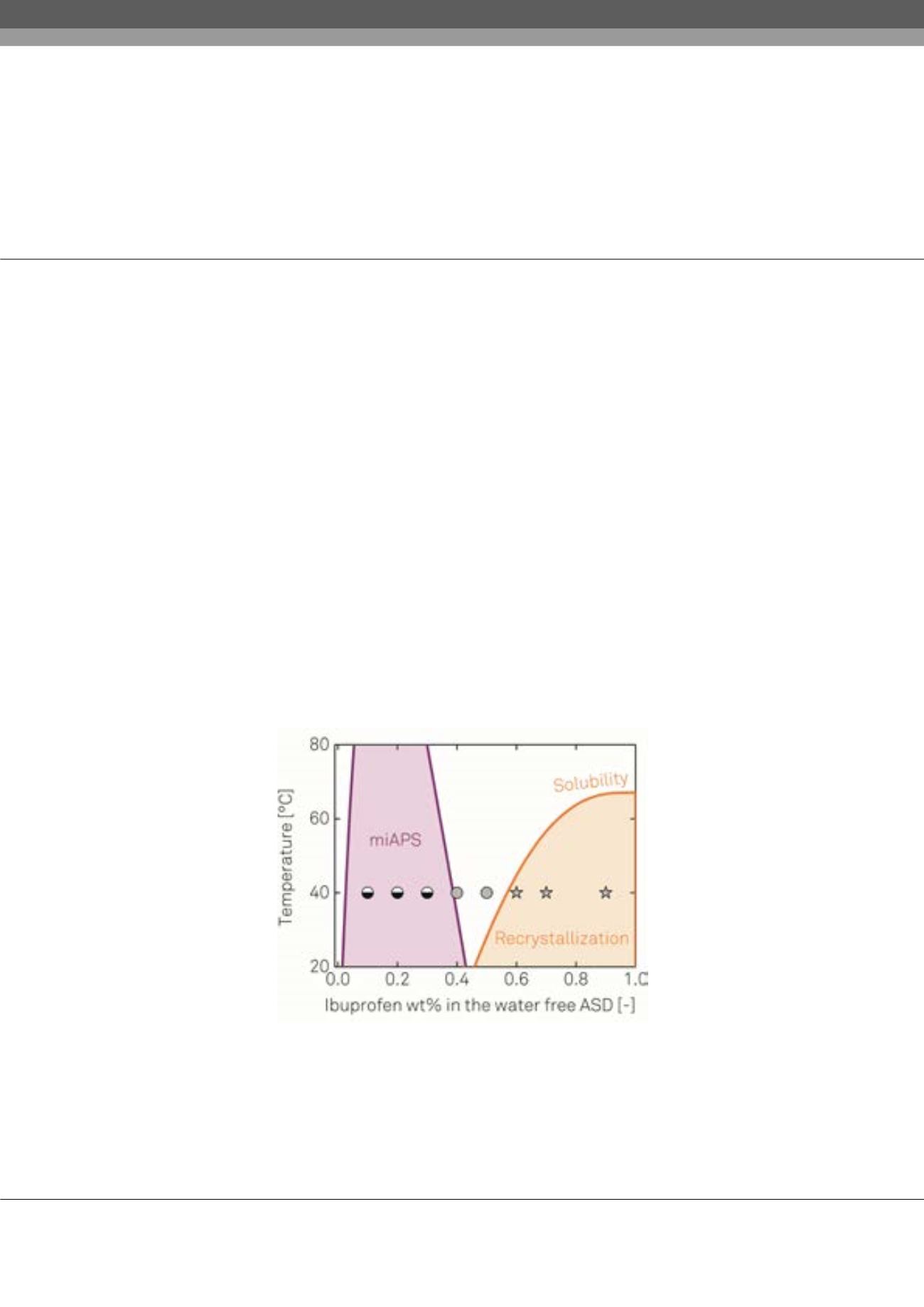
umerous recently-developed Active Pharmaceutical Ingredients (APIs) have a low solubility in water leading to
insufficient absorption and bioavailability. To overcome this solubility limitation, APIs are molecularly dispersed in
hydrophilic polymers. The resulting formulations are denoted as Amorphous Solid Dispersion (ASDs). For the administration
of new pharmaceutical formulations, long-term stability tests are imposed by regulatory authorities at defined conditions
of temperature and humidity (25°C, 60% relative humidity (RH) for 12 months tests and 40°C, 75% RH for accelerated six-
months tests). Recrystallization of the amorphous API and/or moisture-induced amorphous-amorphous phase separation
(miAPS) might occur during storage indicating the thermodynamic instability of the ASDs. Long-term stable formulations
are nowadays identified by trial-and-error principles. The aim of this work was to a-priory estimate the long-term stability
of ASDs by applying advanced thermodynamic methods1 and thus to reduce the experimental effort for finding promising
polymeric carriers suitable for formulation development. In order to validate the thermodynamic predictions, ASDs with
different API/polymer compositions were prepared and subjected to two years enduring long-term stability tests at the
aforementioned conditions. Recurring PXRD measurements were performed to detect recrystallization and Raman mapping
was applied to quantify miAPS. Water sorption was observed as function of time using a magnetic suspension balance. Water
sorption and thereby induced phase transitions (recrystallization/ miAPS) could be predicted in quantitative agreement with
the experimental data. This study showed that results of long-term stability tests can be predicted correctly in early stages of
drug development and that promising polymer candidates for long-term stable ASDs can be identified prior to long-term
stability tests by thermodynamic modeling.
Biography
Christian Luebbert graduated in Chemical Engineering at TU Dortmund University, Germany in 2014. During his work as Research Assistant at the Laboratory of
Thermodynamics, he focuses on the physical long-term stability of amorphous pharmaceutical formulations. With his expertise, he contributes from an engineering
point of view to pharmaceutically highly relevant development of formulation strategies for poorly water-soluble drugs.
Christian.luebbert@bci.tu-dortmund.deChristian Luebbert et al., Insights in Analytical Electrochemistry, 3:2
DOI: 10.21767/2470-9867-C1-002
















