
Notes:
Volume 3, Issue 2
Insights in Analytical Electrochemistry
ISSN: 2470-9867
Analytical Chemistry-Formulation 2017
August 28-30, 2017
Page 46
8
th
Annual Congress on
&
14
th
International Conference and Exhibition on
August 28-30, 2017 Brussels, Belgium
Analytical and Bioanalytical Techniques
Pharmaceutical Formulations
Synthesis of versatile aza-heterocyclic compounds by three component ring transformation
Nagatoshi Nishiwaki
Osaka Kyoiku University, Japan
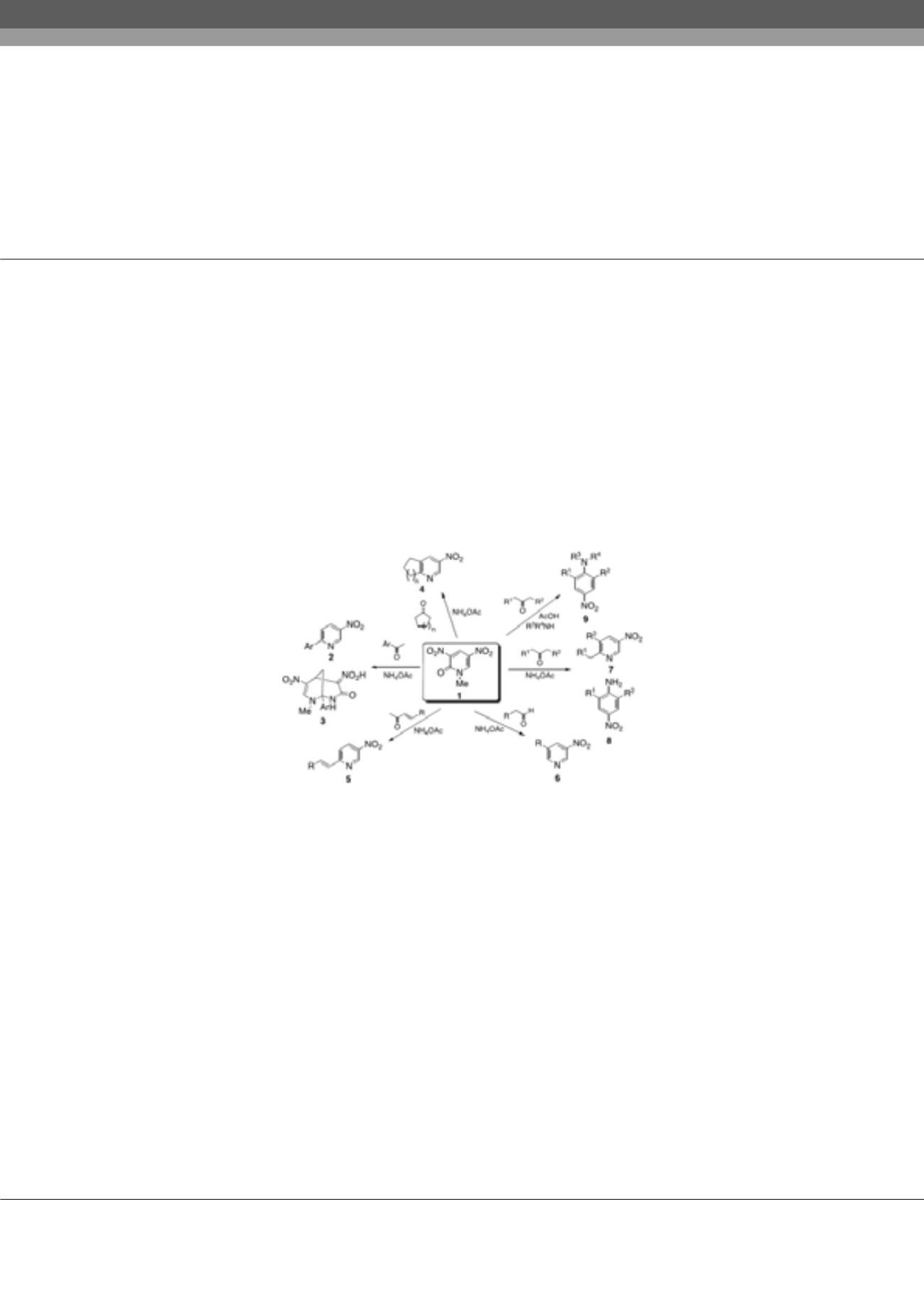
D
initropyridone 1 is an excellent substrate for the nucleophilic type ring transformation to afford heterocyclic compounds
and nitroanilines those are not easily available by alternative methods. When pyridone 1 was reacted with aromatic ketone
in the presence of NH
4
OAc, 6-arylated 3-nitropyriines 2 were formed besides bicyclic compounds 3. This method was also
applicable to synthesis of cycloalka[b]pyridines 4 and 6-alkynylated/alkenylated pyridines 5, respectively. It was found to be
possible to use aldehydes as the substrate, which leading to 3,5-disubstituted pyridines 6. On the other hand, when aliphatic
ketones were employed as the substrate, two kinds of ring transformation proceeded. Namely, 2,6-disubstituted 4-nitroanilines
8 were formed in addition to nitropyridines 7. It was successful to apply this protocol to synthesis of N,N,2,6-tetrasubstituted
nitroanilines 9 upon treatment of dinitropyridone 1 with ketone and amine in the presence of acetic acid.
Biography
Nagatoshi Nishiwaki received his PhD from Osaka University in 1991. He worked at Osaka Kyoiku University (1991–2008). From 2000 to 2001, he joined Karl Anker
Jørgensen’s group at Aarhus University, Denmark. Between 2008 and 2009, he worked at Anan National College of Technology. He then moved to the School of
Environmental Science and Engineering, Kochi University of Technology, in 2009, and he became a Professor in 2011. His research interests comprise synthetic
organic chemistry using nitro compounds, heterocycles (ring transformations, 1,3-dipolar cycloadditions etc.), and pseudo- intramolecular reactions. He has more
than 120 papers and 20 review articles.
nishiwaki.nagatoshi@kochi-tach.ac.jpNagatoshi Nishiwaki, Insights in Analytical Electrochemistry, 3:2
DOI: 10.21767/2470-9867-C1-002
















