
Page 34
E u r o s c i c o n C o n f e r e n c e o n
Physical Chemistry and
Analytical Separation Techniques
October 08-09 , 2018
Amsterdam, Nether l ands
Journal of Organic & Inorganic Chemistry
ISSN: 2472-1123
Physical Chemistry and Analytical Separation Techniques 2018
C
ost-effective solar water splitting requires earth abundant photocatalytic materials converting photons to working electrons
in a highly efficient manner. To develop such suitable photocatalysts, their atomic structure control is of primary importance
since their intrinsic attributes (e.g., electronic band structure, electric properties, catalytic activity, etc.) are governed by their
atomic configuration. In this regard, BiVO
4
’s atomic structure has been engineered via P5+ doping and In
3
+/Mo
6+
dual doping.
The significantly enhanced photo-responsive characteristics of the doping-treated BiVO
4
have been systematically studied within
experimental and theoretical domains. Specifically, VO4 and PO
4
oxoanion exchange in monoclinic BiVO
4
significantly reduces its
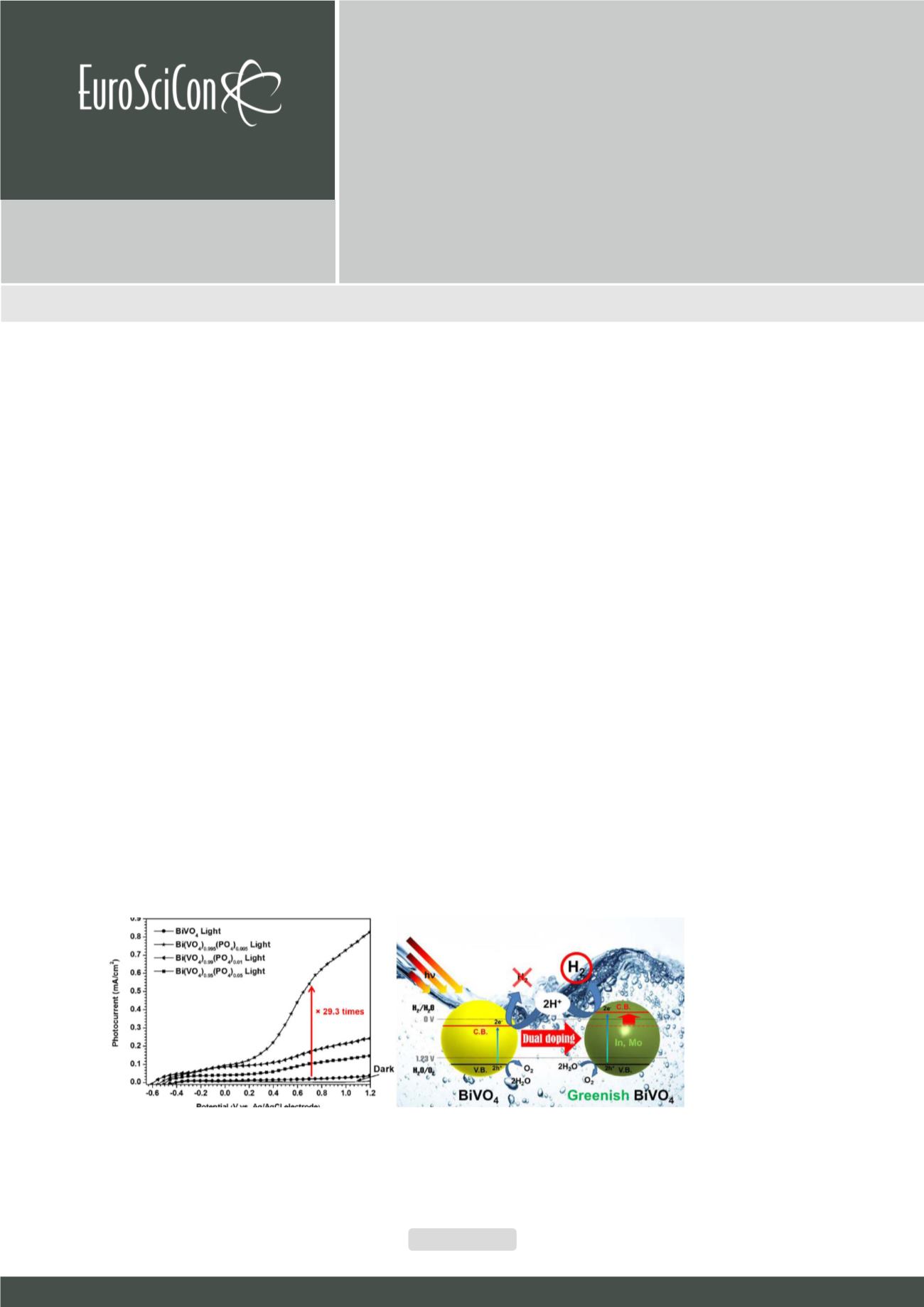
charge-transfer resistance by increasing charge-carrier density, and thus enhances solar-to-hydrogen efficiency up to 29.3 times,
as Fig. 1 shows. Notably, this brand-new oxoanion exchange technique can be applied to other various VO4-based semiconductors
to improve their electronic, catalytic and photochemical properties. To upgrade the photocatalytic performance of BiVO4 further,
its electronic band structure was engineered by simultaneously substituting In
3+
for Bi
3+
and Mo
6+
for V
5+
, which induced partial
phase transformation from pure monoclinic BiVO
4
to a mixture of monoclinic and tetragonal BiVO
4
. This In
3+
/Mo
6+
doped BiVO
4
has a slightly larger band-gap energy (Eg ~2.5 eV) than usual ‘yellow’ monoclinic BiVO4 (Eg ~2.4 eV) and higher (more negative)
conduction band edge (-0.1 VRHE at pH 7) than H+/H
2
potential (0 VRHE at pH 7). Consequently, as Fig. 2 displays, the In3+/Mo
6+
doped BiVO
4
is able to split water into H
2
and O
2
under visible-light irradiation without using any sacrificial reagents (e.g. CH
3
OH
or AgNO
3
). This outcome is the first example of a pure water-splitting photocatalyst responding to visible light without any noble-
metal co-catalyst.
wonjunjo@mit.eduPhase transition induced band structure
engineering of BiVO4 for solar fuel production
Won Jun Jo
1, 3
, Karen K Gleason
1
and Jae Sung Lee
2
1
Massachusetts Institute of Technology, USA
2
Ulsan National Institute of Science and Technology, Republic of Korea
3
Lawrence Berkeley National Laboratory, USA
J Org Inorg Chem 2018 Volume: 4
DOI: 10.21767/2472-1123-C6-018
Fig 1:
Enhanced photocurrent by PO4 doping into BiVO4
Fig 2:
Overall water splitting reaction by In3+/Mo6+ doped BiVO4
















