
Page 96
conferenceseries
.com
Volume 3, Issue 4
J Clin Epigenet
ISSN: 2472-1158
Epigenetics 2017
November 06-08, 2017
EPIGENETICS & CHROMATIN
November 06-08, 2017 | Frankfurt, Germany
2
nd
International Congress on
Preliminary results of continuation maintenance treatment with pemetrexed in advanced non-
squamous non-small cell lung cancer (NSCLC) patients after prior induction chemotherapy– single-
arm phase II study
Nesreen Mohamed Sabry Afifi Mattar
Tanta University, Egypt
Statement of the Problem
: Lung cancer remains one of the leading causes of cancer-related death worldwide. Extending the
duration of treatment with the initial platinum based chemotherapy beyond four to six cycles has been evaluated. The aim of
this study is to investigate efficacy and toxicity of continuation maintenance treatment with pemetrexed (Alimta) in patients
displaying disease control after four cycles of induction with cisplatin plus pemetrexed in advanced non-squamous (NSCLC).
Methodology & Theoretical Orientation
: Between April 2013 and April 2015, 16 patients with pathologically proven stage
III/IV, non-squamous NSCLC, in Clinical Oncology Department, Tanta University Hospital and Tanta Insurance Hospital who
had received prior four cycles of induction with cisplatin (75 mg/m2) plus pemetrexed (500 mg/m2) every 21 days without
disease progression were enrolled. Patients received continuation maintenance treatment with pemetrexed (500 mg/m2, every
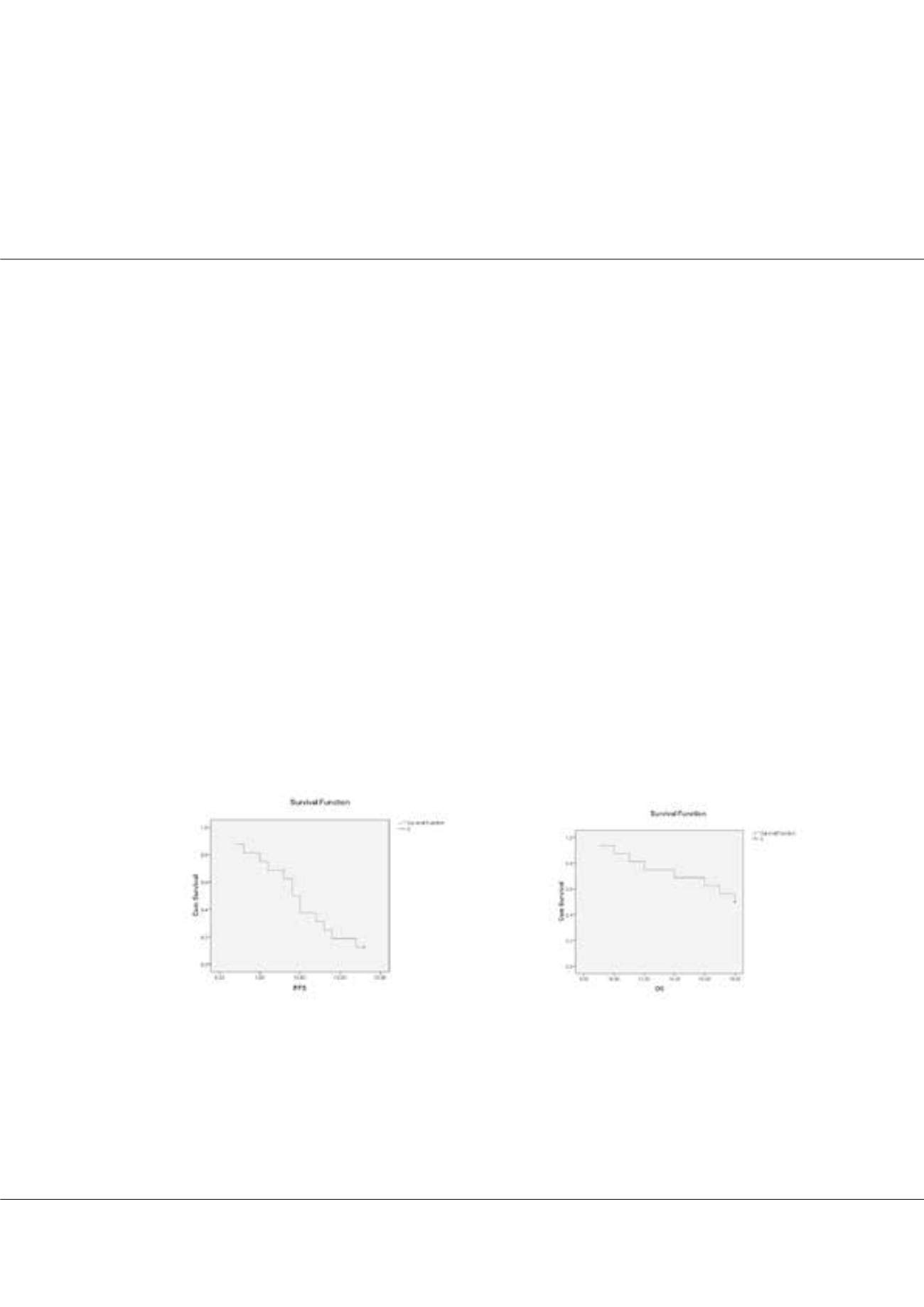
21 days). The primary endpoints of the study were the overall survival and progression-free survival and the secondary end
point was the safety profile.
Finding
: A total of 64 chemotherapy cycles of continuation maintenance pemetrexed were administered. Patients were treated
with a median number of 4 cycles (range 2-30 cycles). Two patients received no more than 2 cycles due to rapid disease
progression. The estimated median PFS and OS were 7.5 and 17 months, respectively. Treatment-related adverse events were
manageable with only 1 patient (6.25%) suffered from Grade 3 anemia and another 1 patient (6.25%) suffered from Grade 4
neutropenia. All patients received full doses of pemetrexed throughout the study. There was no treatment-related death.
Conclusion & Significance
: Using the continuation maintenance regimen with pemetrexed preceded by four cycles of
induction with cisplatin plus pemetrexed represents an obvious treatment advance with an acceptable clinical profile for
patients with non-squamous NSCLC.
Figure 1: Kaplan–Meier curve of progression-free survival. Figure 2: Kaplan–Meier curve of overall survival.
Biography
Nesreen Mohamed Sabry Afifi Mattar works as a Lecturer of Clinical Oncology at Tanta University Hospital and as a Consultant in Insurance Hospital. He has
experience in Teaching to the Postgraduates. He completed his Master Degree in comparative study between chemoradiation and surgery in bladder cancer. His
MD was about comparative study between R-CHOP and CHOP in DLBC NHL according to biomarker mutation (bcl2, p53). Recently, he has published a paper
about the role of lapatinib in combination with letrozole in postmenopausal breast cancer women.
nesreensabry1eg@yahoo.comNesreen Mohamed Sabry Afifi Mattar, J Clin Epigenet 2017, 3:4
DOI: 10.21767/2472-1158-C1-003
















