
Page 98
conferenceseries
.com
Volume 3, Issue 4
J Clin Epigenet
ISSN: 2472-1158
Epigenetics 2017
November 06-08, 2017
EPIGENETICS & CHROMATIN
November 06-08, 2017 | Frankfurt, Germany
2
nd
International Congress on
Investigating the role of nitric oxide on DNAmethylation in breast cancer cells
Berna Demircan Tan
1
, Burcu Yucel
1
, Stephen J Green
2
and James Radosevich
3
1
Istanbul Medeniyet University, Turkey
2
University of Illinois, USA
Introduction & Aim:
Breast cancer is a predominant neoplastic disease among women and regardless of the disease subtypes
it has been proposed that genetic mutations and epigenetic alterations caused by environmental factors may affect tumor
development and growth. Nitric Oxide (NO), a free radical, is a well-known antioxidant has various roles in normal physiology.
However, NO is also an important element in tumor microenvironment and has been linked to tumor growth. NO production
is elevated in various human tumors including breast cancer. NO dependent gene regulation and histone methylation has been
shown in cancer cells. As NO promoted deamination of 5-meC, it can be hypothesized that NO exposure can induce C-T
transition in cancer cells. To exploit this hypothesis, we aimed to evaluate gene promoter methylation of BT-20, T42D and
MCF-7 cell lines upon NO treatment.
Materials & Methods:
Placental cell line was used for normal cell control. E-Cadherin (ECAD), Deleted in Colon Cancer
(DCC), Breast Cancer 1 (BRCA1), Secreted Frizzled Related Protein 1 (SFRP1), Ras-association domain family 1 (RASSF1A),
O-6-Methylguanine-DNA Methyltransferase (MGMT) promoter methylations were assessed before and upon no treatment
using ion torrent next-generation sequencing system after bi-sulfite conversion. Promoter methylation was determined as
percentage of cytosine reads of the total cytosine and thymine reads of each CpG site. To compare differences between groups,
student t-test was applied.
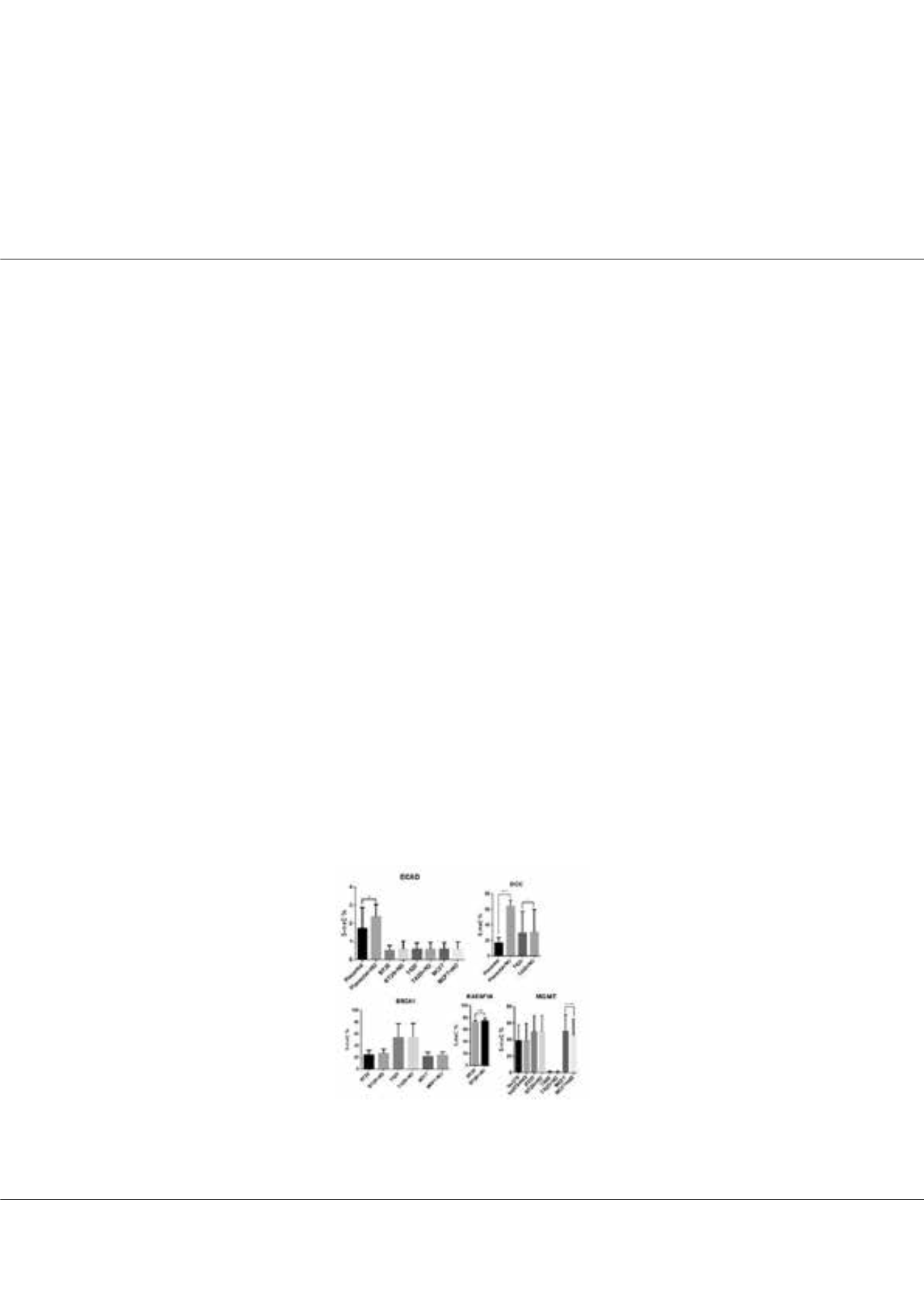
Results:
In control cell line, the effect of NO on DNA methylation was evaluated only for ECAD and DCC genes as the reading
counts were below 100 for other genes. We found that NO exposure increased promoter methylation percentage of ECAD
and DCC genes in placental cells (p<0.05). However, no significant change was seen on other cell lines for ECAD. DCC gene
promoter methylation was found higher in T42D cells compared to placental cells and the methylation was increased upon
NO exposure in both cell lines (p<0.05). We didn’t find any significant chance in BRCA1 gene promoter methylation upon
NO treatment in all cell lines. RASSF1A gene methylation in BT-20 cells and SFRP1 gene methylation in T42D cells were
significantly increased upon NO treatment (p<0.05).
Conclusion:
Our results can be further expanded using different cancer cell lines and interpreting the gene expression levels.
We believe our results will contribute to the studies to further investigate the role of NO in regulation of gene expression in
cancer cells.
Biography
Berna Demircan Tan has completed her PhD degree in Biochemistry. She completed her Postdoctoral training in USA in 2006-2010. Her research efforts have
focused on the epigenetic basis of cancer, particularly DNA methylation. She has publications and book chapters on her research field. Currently, she is working as
an Associate Professor at Istanbul Medeniyet University, Istanbul, Turkey.
berna.demircan@medeniyet.edu.trBerna Demircan Tan et al., J Clin Epigenet 2017, 3:4
DOI: 10.21767/2472-1158-C1-003
















