
Notes:
Volume 3, Issue 2 (Suppl)
Trends in Green chem
ISSN: 2471-9889
Environmental & Green Chemistry 2017
July 24-26, 2017
Page 25
5
th
International Conference on
6
th
International Conference on
July 24-26, 2017 Rome, Italy
Environmental Chemistry and Engineering
Green Chemistry and Technology
&
A single enzymatic approach for deracemization of secondary alcohols
Musa M Musa
1
, Ibrahim Karume
1
and
Samir Hamdan
2
1
King Fahd University of Petroleum and Minerals, Saudi Arabia
2
King Abdullah University of Science and Technology, Saudi Arabia
C
ontrolling enantioselectivity of alcohol dehydrogenase-catalyzed transformations using site-directed mutagenesis enabled their
used in racemization of enantiopure secondary alcohols and in deracemization of racemic secondary alcohols. Controlled
racemization of enantiopure secondary alcohols is achieved using various mutants of secondary alcohol dehydrogenase from
Thermoanaerobacter ethanolicus
(TeSADH) and in the presence of the reduced and oxidized forms of its cofactor nicotinamide-
adenine dinucleotide. We also developed a deracemization method for secondary alcohols that uses a single mutant of TeSADH
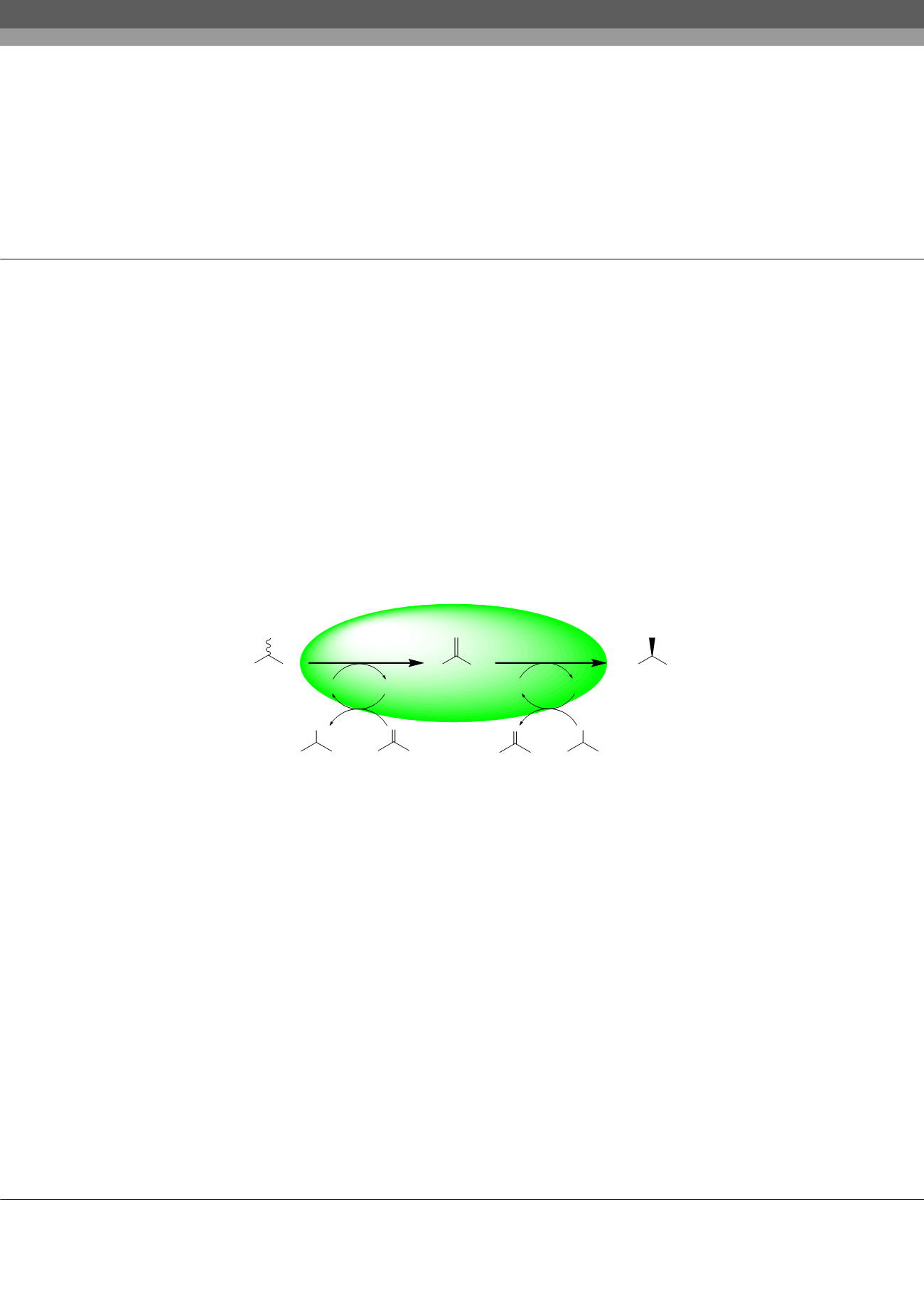
in two steps. A single mutant of TeSADH enables the non-stereoselective oxidation of racemic alcohols to ketones, followed by a
stereoselective reduction reaction for the resulted ketone. The key component in this deracemization approach is the ability to control
the TeSADH-catalyzed transformations using protein engineering and medium engineering. Varying the amounts of acetone and
2-propanol co-substrates controls the stereoselectivities of the consecutive oxidation and reduction reactions, respectively. We used
one enzyme to accomplish deracemization of secondary alcohols with up to >99% ee and >99% recovery in one pot and without the
need to isolate the prochiral ketone intermediate. This deracemization approach is simple, efficient and environmentally benign.
Figure 1:
Single enzymatic approach for secondary alcohols using
Thermoanaerobacter ethanolicus
secondary alcohol dehydrogenase.
Biography
Musa M Musa received his PhD from the University of Georgia under the supervision of Prof. Robert S Phillips working in biotransformation in non-aqueous media.
He then carried research with Prof. Mark D Distefano as a Postdoctoral Associate at University of Minnesota, where he focused on synthesis and evaluation of
protein farnesyltransferase inhibitors and protein labeling. In 2009, he joined King Fahd University of Petroleum and Minerals (KFUPM) as Assistant Professor. He is
currently an Associate Professor of Chemistry at KFUPM. His research interests include employing enzymes in organic synthesis. More specifically, he is interested
in enzyme-catalyzed racemization, deracemization, and dynamic kinetic resolution of alcohols.
musam@kfupm.edu.saMusa M Musa et al., Trends in Green chem, 3:2
DOI: 10.21767/2471-9889-C1-002
" non
-
s t ereose l ec ti ve
ox id a ti on con dti ons "
" s t ereose l ec ti ve
re d uc ti on con dti ons "
p roc hi ra l k e t one
R acem i c
a l co h o l s
E nan ti o p ure
a l co h o l s
T e SADH
T e SADH
R
1
R
2
OH
R
1
R
2
OH
R
1
R
2
O
NADP
+
NADPH
OH
O
NADP
+
NADPH
OH
O
3%
v / v
30%
v / v
















