
Notes:
Volume 3, Issue 2 (Suppl)
Trends in Green chem
ISSN: 2471-9889
Environmental & Green Chemistry 2017
July 24-26, 2017
Page 80
5
th
International Conference on
6
th
International Conference on
July 24-26, 2017 Rome, Italy
Environmental Chemistry and Engineering
Green Chemistry and Technology
&
Chemoselective hydrodehalogenation and high efficiency birch reduction using two-dimensional inorganic
electride dicalcium nitride ([Ca
2
n] +∙e-) as a reducing agent
Byung Il You, Ye Ji Kim
and
Sung Wng Kim
Sungkyunkwan University, Republic of Korea
P
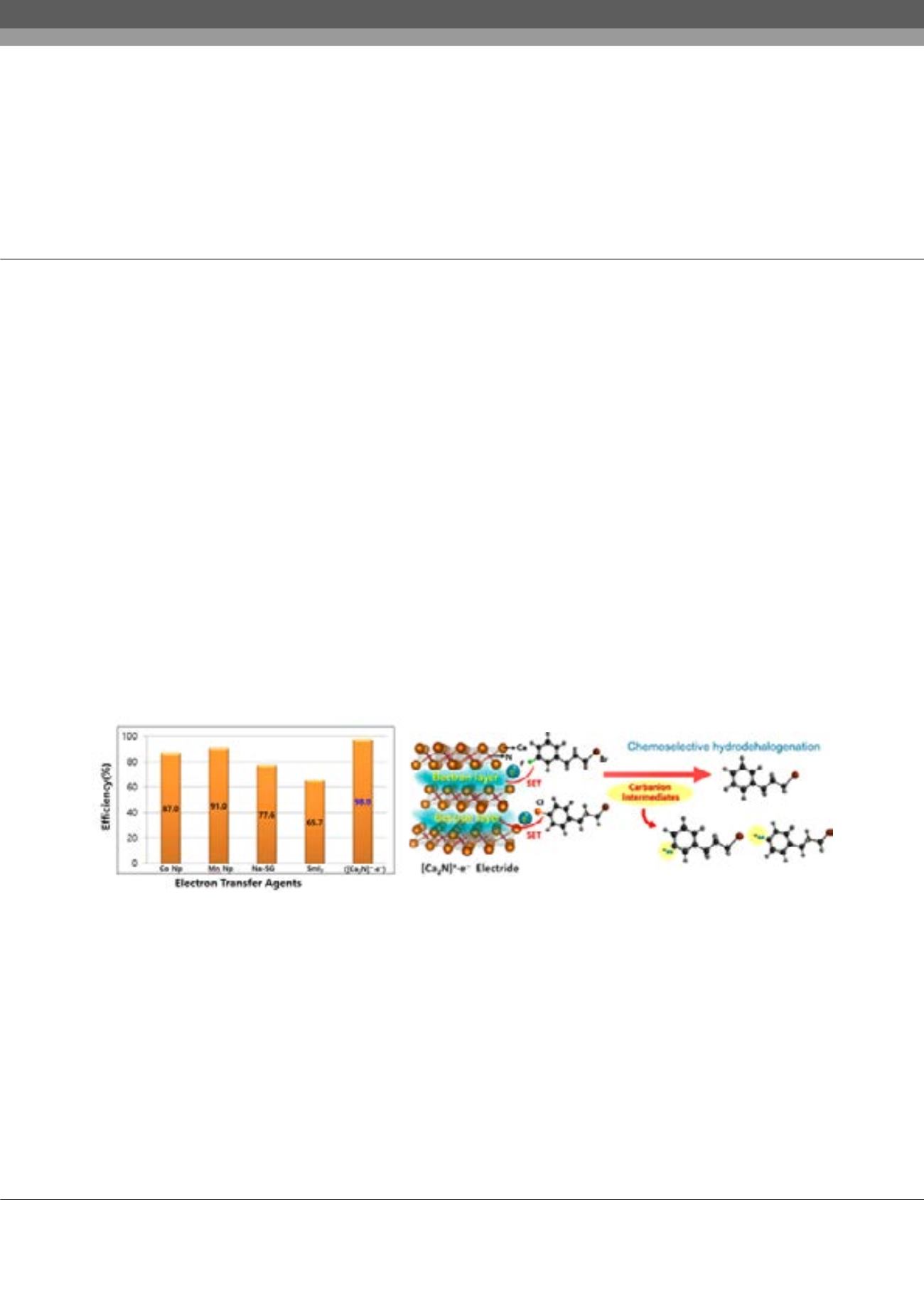
olycyclic aromatic and halogenated organic compounds are known as a functional material which has applications in chemical
industry, biology, pharmacology. Inspite of the utility of polycyclic aromatic and halogenated hydrocarbons, it has concerns about
human health such as carcinogenic or mutagenic risk and considerable environmental pollution. In hydrogenation of polycyclic
aromatic hydrocarbons (Birch reduction) and dehalogenation reactions, the consecutive single-electron transfer from reducing
agents generates the radical and corresponding carbanion and removes the halogen atom and π–conjugated electron in polycyclic
aromatic compounds. The most prominent feature of two-dimensional electride [Ca
2
N] +·e
-
- is powerful electron donating nature
as reductant originated from high electron concentration and low work-function. The electron donating ability of two-dimensional
electride was demonstrated through single electron transfer involving chemical reactions such as pinacol coupling reaction,
trifluoromethylation, transfer hydrogenation. Herein, we report a new strategy for efficient chemoselective hydrodehalogenation
through the formation of stable carbanion intermediates, which are simply and birch reduction of polycyclic aromatic rings by using
the anionic electrons of two dimensional inorganic electride [Ca
2
N]+·e
-
-with effective electron transfer ability. The consecutive single-
electron transfer from inorganic electride [Ca
2
N] +·e
-
- stabilized free cabanions, which is a key step in achieving the selective reaction.
The control of equivalent of inorganic electride [Ca
2
N] +·e
-
-and reaction condition provided exceptional reactivity in comparison
with other reducing agents such as cobalt nanoparticle, manganese nanoparticle, Samarium iodide (SmI
2
) and sodium silica-gel.
Also, a determinant more important than leaving group ability is the stability control of free carbanions according to the s character
determined by the backbone structure. We anticipate that this approach may provide new insight into selective chemical formation,
including hydrodehalogenation.
Biography
Byung Il Yoo got his Bachelor’s degree in Department of Chemistry from Korea Advanced Institute of Science and Technology in 2014. Since 2016, he joined prof.
Sung Wng Kim’s group of Sungkyunkwan University as Post-Graduate student. He research interest includes synthesis new type of inorganic electride, chemical
application of inorganic electride.
ybi9002@skku.eduByung Il You et al., Trends in Green chem, 3:2
DOI: 10.21767/2471-9889-C1-002
















