
Page 116
Nano Research & Applications
ISSN 2471-9838
E u r o S c i C o n C o n f e r e n c e o n
Nanotechnology &
Smart Materials
O c t o b e r 0 4 - 0 6 , 2 0 1 8
Am s t e r d a m , N e t h e r l a n d s
Nanotechnology & Smart Materials 2018
Effect of synthetic conditions on the textural properties of
gluconic acid coated magnetic iron oxide nanoparticles
Bamidele M Amos-Tautua
1, 2
, Sandile P Songca
3
and
Samuel O Oluwafemi
1, 2
1
University of Johannesburg, Doornfontein Campus, South Africa
2
CNRS-University of Johannesburg, South Africa
3
University of Zululand, South Africa
A
pplications of magnetic iron oxide-based nanomaterials are desirable for removal of heavy metals and other contaminants
from waste water because of their important features such as small particle size, high surface area, saturated magnetism,
definite pore size and pore volume. This study tends to evaluate the effect of synthetic conditions such as temperature, volume
and concentration of precursors on the textural properties of gluconic acid coated magnetic iron oxide nanoparticles. Iron
III salt and glucose were used as precursors to prepare the nanomaterials and were characterized using Fourier transform
infrared spectroscopy (FTIR), scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDX), X-ray powder
diffractometry (XRD) and thermal gravimetric analysis (TGA). The magnetic properties of the as-synthesized materials were
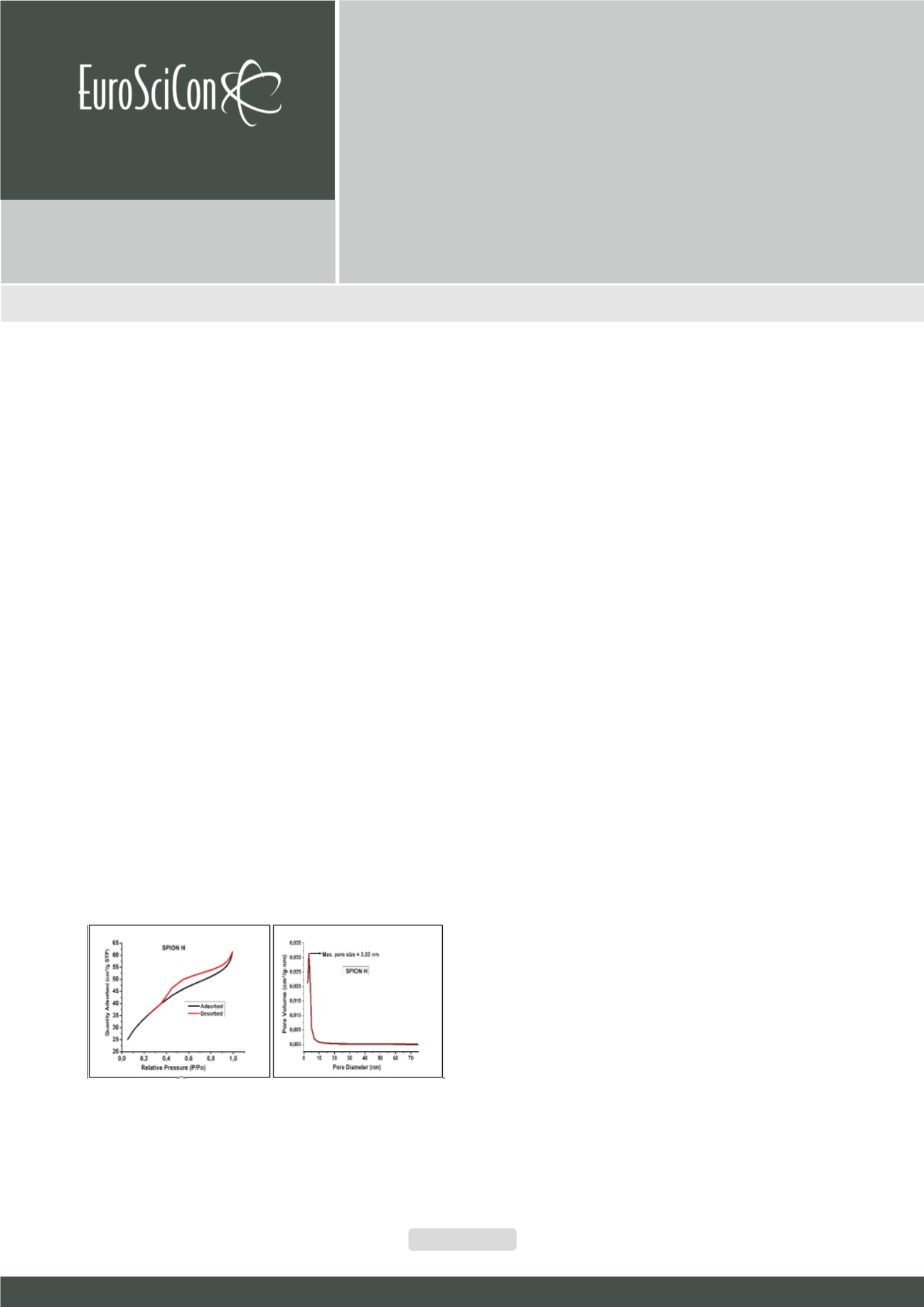
measured using the SQUIDmagnetometer. Textural properties including surface areas, average pore size and pore size distribution
of the studied samples were determined by the Brunauer–Emmett–Teller (BET) method. Results showed that the synthesized
samples are paramagnetic and superparamagnetic in behaviour. In addition, the mean pore size of the synthesized samples varies
between 3.33 and 7.59 nm. This shows that the nanomaterials are mesoporous in nature and contain large plate-like particles
with slit-shaped pores which favour adsorption of waste materials from contaminated water. Surface area of the nanomaterials
ranged between 158 and 353.80 m
2
/g which are far higher than values of the standard magnetic iron oxides reported in literature.
The as-synthesized magnetic iron oxide nanomaterials in this study are considered as good candidates for applications such as
adsorbents for removal of heavy metals and other organic pollutants from waste waters, photo-electrochemical cells, catalysts
and sensors.
bweinpere@yahoo.comNano Res Appl Volume:4
DOI: 10.21767/2471-9838-C6-025
















