
Structural Chemistry & Crystallography Communication
ISSN: 2470-9905
June 04-05, 2018
London, UK
Crystallography 2018
Page 12
3
rd
Edition of International Conference on
Advanced Spectroscopy,
Crystallography and Applications
in Modern Chemistry
T
ransition Metal Oxides with strongly correlated electrons
have been studied intensively due their interesting physical
properties. This includes colossal magnetoresistance (CMR)
where huge variations in resistance are achieved just by small
changes in the applied magnetic field, or high temperature
superconductivity (HTC) to name two of them [3-6]. These
materials are characterized by the existence of several
competing states such as charge, spin and orbital ordering,
interacting in a synergetic way and leading to fairly complex
phase diagrams. Thereby the physical properties can be tuned
in a wide range via hole doping, e.g. by cation substitution as
is the case for
RE2-xSrxMO4.Analternative way of hole doping
presents oxygen intercalation, generally proceeding at ambient
temperature via a topotactic oxygen uptake along shallow
potential diffusion pathways. Contrary to the cation substitution,
requiring high reaction temperatures, oxygen intercalation
reactions allow the controlled synthesis of strongly correlated
oxides far away from thermodynamic equilibrium, essentially
resulting in kinetically stabilized and thus metastable phases.
Low temperature reactivity of solids may thus be used as a
concept, to investigate the limits of available structural and
electronic complexity in transition metal oxides. The reaction
pathway to insert oxygen at low temperatures in solid oxides
becomes a decisive parameter to tune correlations, leading to
extremely complex phase relations as physical and structural
properties are not only depending on the overall stoichiometry,
but decisively on the sample history. Taking these oxides as
oxygen ‘sponges’ operating at low reaction temperatures down
to ambient, structural and electronic correlation lengths could
then be influenced by the reaction conditions and kinetics. We
here discuss here the challenges, low temperature solid state
reactivity implies for the synthesis of new complex oxides but
equally the current understanding of the relying oxygen diffusion
mechanisms, having a huge fundamental and technological
interest.
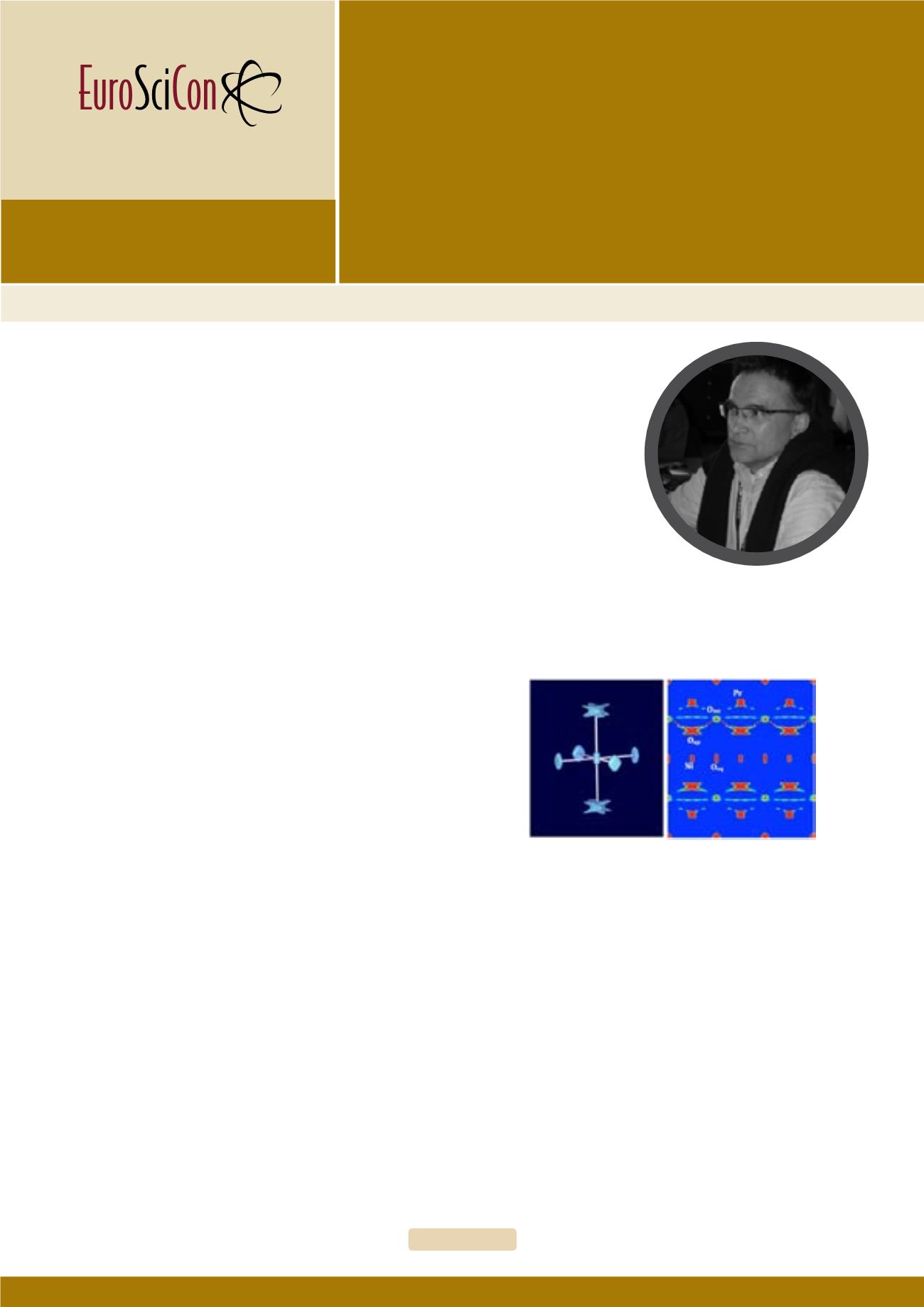
Pr2NiO4.25: Representations of the NiO6 isosurfaces (left) for indicate the
anharmonic double potential of the apical oxygen atoms present at 673
K, obtained from single crystal neutron diffraction and Maximum Entropy
Analysis. The large anisotropic displacements of the apical oxygen atoms
along [110] directly point towards the interstitial oxygen sites, forming a
shallow oxygen diffusion pathway which is dynamically activated
Recent Publications
1. From T to T’-La2CuO4 via Oxygen Vacancy Ordered
La2CuO3.5, M. Ikbel Houchati, M. Ceretti, C. Ritter and
W. Paulus, Chem. Mater. 2012, 24, 3811-3815
2. One-dimensional oxygen diffusion mechanism in
Sr2ScGaO5 electrolyte explored by neutron and
synchrotron diffraction, 17O-NMR andDFTcalculations,
S. Corallini M. Ceretti, G. Silly, A. Piovano, S. Singh, J.
Stern, C. Ritter, J. Ren, H. Eckert, K. Conder, Wei-tin
Chen, Fang-Cheng Chou, N. Ichikawa, Y. Shimakawa, W.
Paulus, J. Phys. Chem. C, 2015, 119 (21), 11447–11458
PHONON ASSISTED OXYGEN DIFFUSION VS. OXYGEN
AND ELECTRONIC ORDERING MECHANISMS IN NON-
STOICHIOMETRIC CORRELATED OXIDES
Werner Paulus
University of Montpellier, France
Werner Paulus, Struct Chem Crystallogr Commun 2018, Volume 4
DOI: 10.21767/2470-9905-C1-004
















