
Structural Chemistry & Crystallography Communication
ISSN: 2470-9905
June 04-05, 2018
London, UK
Crystallography 2018
Page 36
3
rd
Edition of International Conference on
Advanced Spectroscopy,
Crystallography and Applications
in Modern Chemistry
O
xygen ion conductors are materials of major interest for a
series of application in the area of solid state ionics (fuel
cells, batteries, electrodes, sensors, catalysts, etc…). In this
respect oxides with brownmillerite type structure (A2BB’O5),
have attracted much attention, especially as they show oxygen
ion mobility down to ambient temperature. This mobility is
a result of a phonon assisted diffusion mechanism, based
on a dynamic oxygen disorder scenario of the infinite BO4
chains [1, 2]. Brownmillerite type frameworks containing
B-cations with saturated or empty electron shells (d0 or d10
configurations) present a special case, as they impose a fixed
oxygen stoichiometry, making them good candidates to study
oxygen diffusion mechanisms on a microscopic level. In this
context, we have synthesized a new phase Sr
2
ScGaO
5
, having
pure oxygen ion conductivity. Depending on the synthesis route,
it shows two polymorphs: orthorhombic Brownmillerite type
structure or an oxygen deficient cubic perovskite structure.
When synthesizing Sr
2
ScGaO
5
by classical solid state reaction
at 1200°C, the thermodynamically stable phase obtained shows
the brownmillerite framework [3]. Heating at higher temperature,
it shows a phase transition to the cubic perovskite structure
completed at 1500°C, associated with improved oxygen ion
conduction [4]. Since the cubic symmetry can be maintained
down to ambient temperature, we were able to grow high
quality single crystal of the cubic phase [5]. We report here on
a combination of characterization on the brownmillerite as
well on the cubic Sr
2
ScGaO
5
. High-resolution structure analysis
has been performed using X-rays (synchrotron and laboratory)
and neutron diffraction methods, combined with NMR analysis
for local environment [3]. In particular, single crystal neutron
diffraction with subsequent analysis of the nuclear scattering
density by the Maximum Entropy Method has been performed
in order to describe in more detail oxygen displacement factors
and associated diffusion pathways [5]. To better understand the
oxygenmobilitymechanisms, these studieswere complemented
by Raman and impedance spectroscopy.
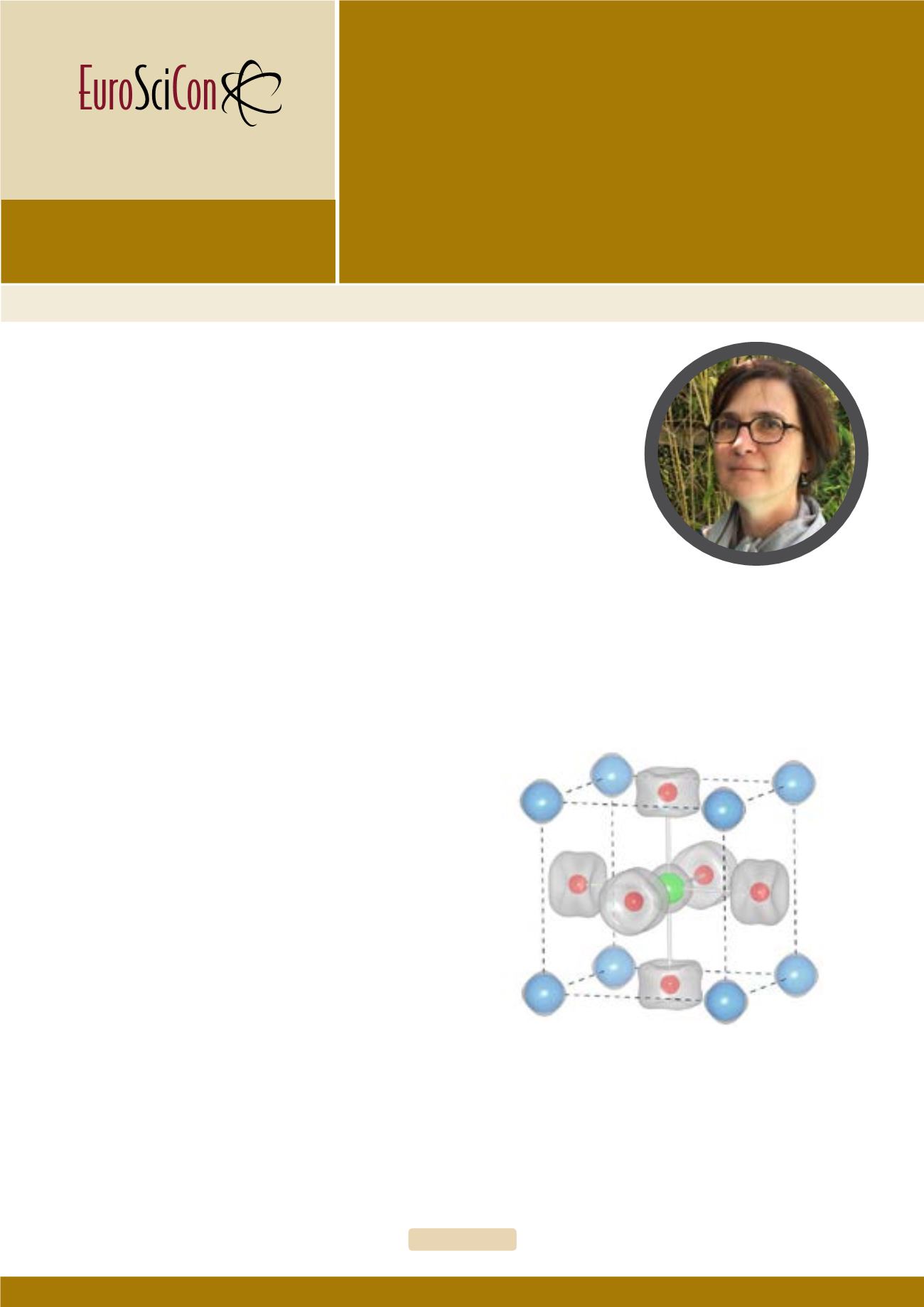
Nuclear scattering density (in grey) of the Sr
2
ScGaO
5
obtained at room tem-
perature from neutron single crystal diffraction and subsequent Maximum En-
tropy reconstruction. The preovskite unit cell and the (Sc/Ga)O6 octahedra are
outlined. While isotropic displacements are found for Sr and (Sc/Ga), oxygen
atoms (in red) show an anisotropic disk shape distribution
Recent Publications
1. Paulus W. et al. (2008) Lattice Dynamics To Trigger
Low Temperature Oxygen Mobility in Solid Oxide Ion
STRUCTURAL FEATURES OF A NEW OXYGEN
DEFICIENT PEROVSKITE SR
2
SCGAO
5
, A PROMISING
OXYGEN ION CONDUCTOR AT MODERATE
TEMPERATURE
Monica Ceretti
Institut Charles Gerhardt Montpellier, France
Monica Ceretti, Struct Chem Crystallogr Commun 2018, Volume 4
DOI: 10.21767/2470-9905-C1-004
















