
Page 36
Notes:
conferenceseries
.com
Volume 3, Issue 2
ISSN: 2470-9905
Crystallography 2017
October 16-17, 2017
2
nd
International Conference on
October 16-17, 2017 | Chicago, USA
Applied Crystallography
Structural study of sulfide glassy electrolytes for all-solid-state batteries
Koji Ohara
1
and
Shinya Shiotani
2
1
Japan Synchrotron Radiation Research Institute, Japan
2
Toyota Motor Corporation, Japan
S
ulfide glass ceramics are of interest for use as solid electrolytes in lithium ion batteries, because the realization of an all-solid-
state battery will enable the miniaturization of battery packages and reduce safety issues. In general, the crystallization of glassy
materials results in a decrease in the conductivity, although it increases in a few sulfide glasses owing to the crystallization of a highly
conductive new phase. Significant progress has been made so far with the discovery of numerous sulfide crystalline compounds
with high ionic conductivities such as Li
7
P
3
S
11
, Li
10
GeP
2
S
12
, Li
7
P
2
S
8
I and Li
10
SnP
2
S. Their conductivities are higher than those of the
corresponding sulfide glasses. Recently, we reported that 75Li
2
S-25P
2
S
5
glass in the binary Li
2
S-P
2
S
5
system with strongly polarized
sulfur has high ionic conductivity. In the sulfide glasses as presented here, an interesting improvement in the conductivity is observed
during annealing, which appears in the glassy phase. In this presentation, we report the local glassy structure with a mixture of
glass and crystalline phases by using synchrotron X-ray pair distribution function (PDF) analysis. The differential pair distribution
function is utilized as a methodology for the mixed materials in our work to extract the glassy structure in the 75Li
2
S-25P
2
S
5
sulfide
glass ceramic. This method quantitatively reproduced the fraction of mixed phases, which was in agreement with the result of NMR.
The extracted glassy structure exhibited no changes during the annealing treatment. Owing to the crystallization of the majority
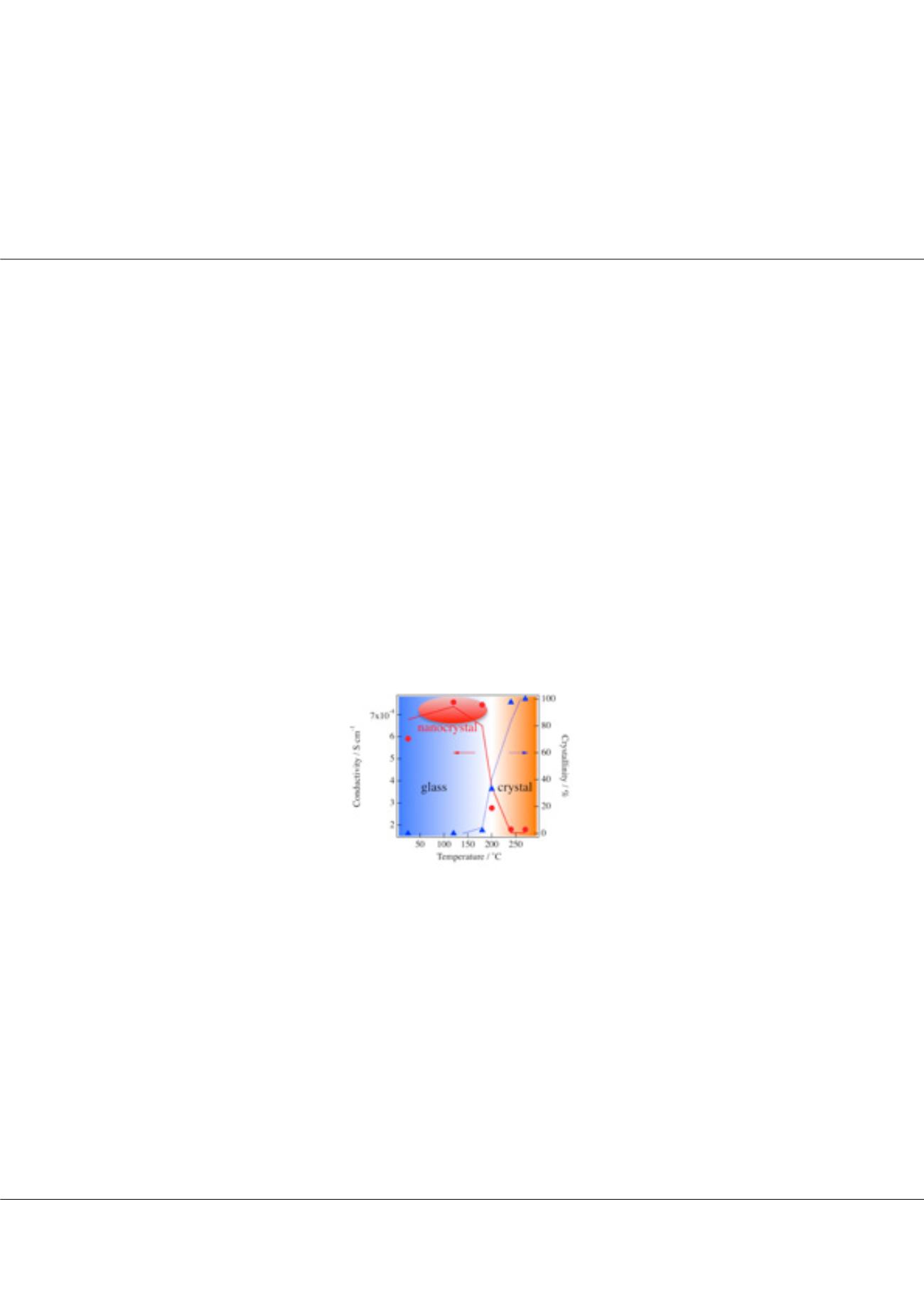
phase, the ionic conductivity in this glass decreased after crystallization. We observed the formation of a nanocrystalline phase in the
diffraction pattern obtained during the annealing. Thus, our present finding of minority component is expected to lead to further
understanding for the development of solid electrolytes with high ionic conductivity.
Figure-1:
Crystallinity obtained from the pair distribution function under each annealing condition and the lithium ionic conductivity.
Biography
Koji Ohara has received his PhD in Condensed Matter Chemistry and Physics from Kyushu University of Japan, working with Prof. S. Takeda. He then did his Post-doctoral
research with S. Kohara at Japan Synchrotron Radiation Research Institute (JASRI), where he studied elemental specific pair distribution function (PDF) analysis using
anomalous X-ray scattering for disordered materials. After two years at JASRI, he moved to the position of Research Assistant Professor at Office of Society-Academia
Collaboration for Innovation of Kyoto University in 2012. He has worked on structural studies of electrolytes in lithium ion batteries. He has been working as a Researcher
at JASRI since 2015.
ohara@spring8.or.jpKoji Ohara et al., Struct Chem Crystallogr Commun, 3:2
DOI: 10.21767/2470-9905-C1-002
















