
E u r o p e a n C o n g r e s s o n
Pharma
American Journal of Pharmacology and Pharmacotherapeutics
ISSN: 2393-8862
A u g u s t 1 3 - 1 4 , 2 0 1 8
P a r i s , F r a n c e
Pharma 2018
Page 19
A
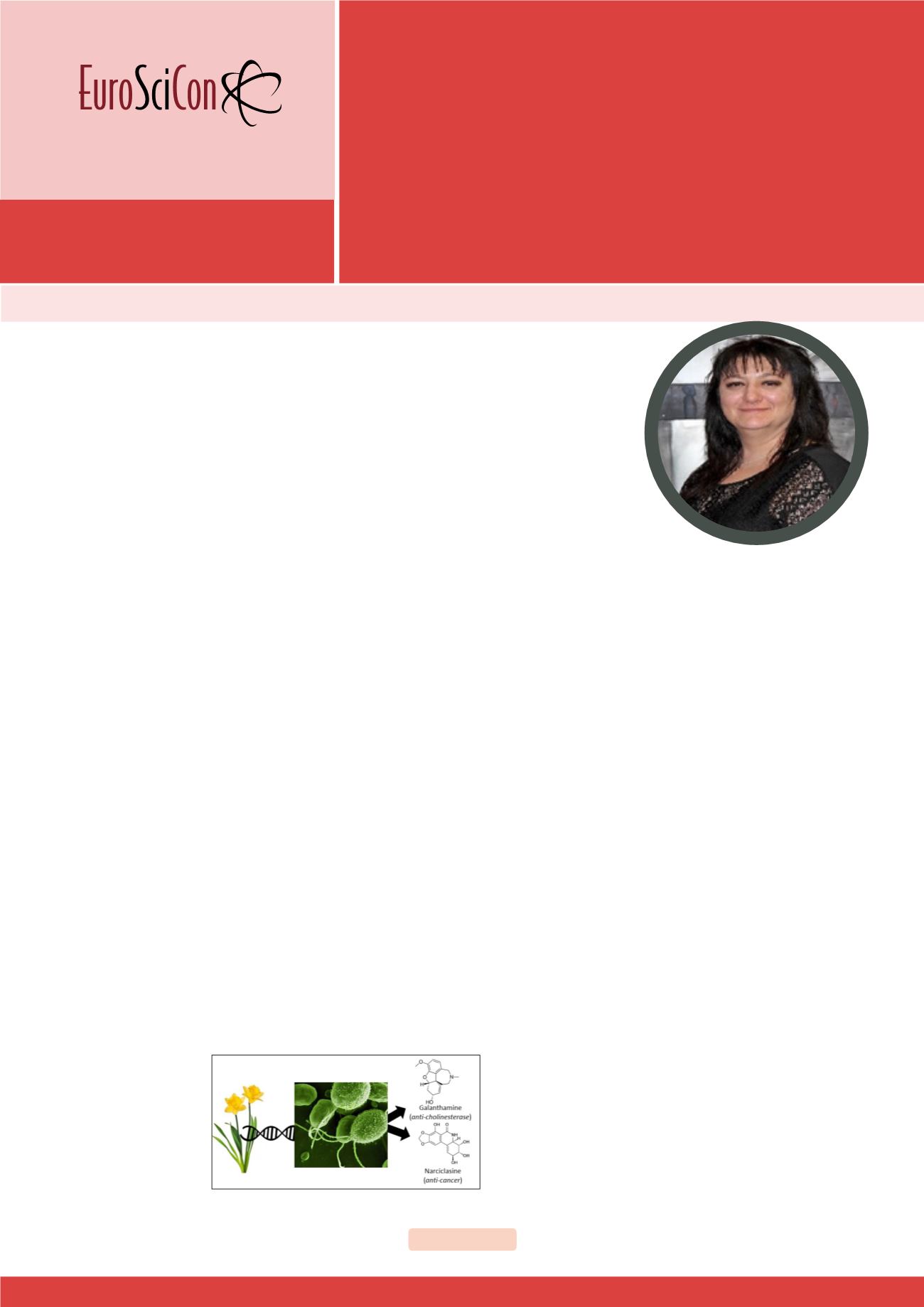
marylllidaceae plant alkaloids (AAs) possess powerful pharmaceutical and
biotechnological properties. AA metabolism and its fascinating molecules,
including anti-acetylcholinesterase galanthamine, anti-microbial lycorine and
anti-cancer narciclasine, have attracted the attention of both the industry and
researchers involved in plant science, chemical bioengineering and medicine.
Currently, access and availability of high-value AAs [commercialized (e.g.
galanthamine) or not (e.g. narciclasine)] is limited by low concentration in
plants, seasonal production and time-consuming low-yield extraction methods.
Nevertheless, commercial AA galanthamine is still extracted from plant
sources. Efforts to improve the production of AA have largely been impaired by
the limited knowledge on AA metabolism. The purpose of this study is to use
recent development and integration of next-generation sequencing technologies
and metabolomics analyses to unravel metabolic pathways allowing the use
of metabolic engineering approaches to increase production of valuable AAs
(Figure 1). Novel genes encoding AA biosynthetic enzymes were identified
from our transcriptome databases using bioinformatics tools. The genes were
characterized and their activities were studied through classical biochemistry
experiment such as cloning into expression vectors, heterologous expression,
recombinant protein purification and enzyme assays. In addition, AA precursor
pathway was introduced into microalgae cells to 1) validate the function of the
biosynthetic genes and 2) to produce AAmolecules. Next, the final steps of the AA
biosynthetic pathway will be added to reach galanthamine or other AA synthesis
in microalgae. Metabolic engineering provides opportunity to overcome issues
related to restricted availability, diversification and productivity of plant alkaloids.
Engineered cells can act as biofactories by offering their metabolic machinery
for the purpose of optimizing the conditions and increasing the productivity of a
specific alkaloid.
Biography
Isabel Desgagne-Penix has completed her PhD in Cell and
Molecular Biology in 2008 from the University of Texas at San
Antonio and Postdoctoral studies in Plant Biochemistry at the
University of Calgary. She has her expertise in Medicinal Plant
Metabolism specifically with molecule of the alkaloid category.
She is the Director of the plant specializedmetabolism research
laboratory. She has published numerous papers in reputed
journals and has been serving as an Editorial Board Member of
the journal of Plant Studies.
Isabel.Desgagne-Penix@uqtr.caMetabolic engineering of microalgae cells
for the production of pharmaceutical
Amaryllidaceae alkaloids
Isabel Desgagne-Penix
Universite du Quebec a Trois-Rivieres, Trois-Rivieres, Canada
Isabel Desgagne-Penix, Am J Pharmacol Pharmacother 2018, Volume 5
DOI: 10.21767/2393-8862-C1-001
















