
Crystallography 2018
Structural Chemistry & Crystallography Communication
ISSN: 2470-9905
Page 43
June 04-05, 2018
London, UK
3
rd
Edition of International Conference on
Advanced Spectroscopy,
Crystallography and Applications
in Modern Chemistry
T
he structural properties of the Ln
4
Al
2
O
9
(Ln=rare-earth)
type phases have attracted attention because of their ionic
conductivity and thermal stability [1-3]. Minerals belonging to
the cuspidine group have the following general stoichiometry:
M
4
(Si
2
O
7
)X
2
(M= divalent cation; X= OH, F, O), with Ca
4
(Si
2
O
7
)
(OH,F)2 being the archetype compound. The cuspidine structure
can be described as built up of chains of edge-sharing MO
7
/
MO
8
polyhedra running parallel to the a-axis (in the P21/c space
group) with tetrahedral disilicate groups, Si
2
O
7
, interconnecting
these ribbons through the vertexes [3]. In more recent years the
preparation and characterization of inorganic oxide fluorides has
attracted significant interest [4]. Given the recent studies on oxide
ion/proton conductivity in La
4
(Ga
2
-xTixO
7
+x/2)O
2
, illustrating the
ability of the cuspidine structure to accommodate extra anions
[5], we have investigated the possible incorporation of fluorine
into Ln
4
Al
2
O
9
to give Ln
4
Al
2
O
9
-xF
2
x (Ln= Sm, Eu, Gd, Tb) (0≤x≤1).
We report here on the results of the fluorination of a range of
cuspidine-related phases of composition Ln
4
Al
2
O
9
(Ln=Sm, Eu,
Gd, Tb). The introduction of fluorine (2F- replace 1O
2
-) is achieved
through a low-temperature (400ºC) reaction with poly(vinylidene
fluoride) (PVDF) or poly(tetrafluoroethylene) (PTFE). We
investigate the effects of fluorination on the starting structure by
X-ray diffraction, Raman spectroscopy and X-ray photoelectron
spectroscopy. The thermal stability of these samples before and
after fluorination was evaluated in air. The starting materials
Ln
4
Al
2
O
9
(Ln=Sm, Eu, Gd, Tb) showed a monoclinic crystal
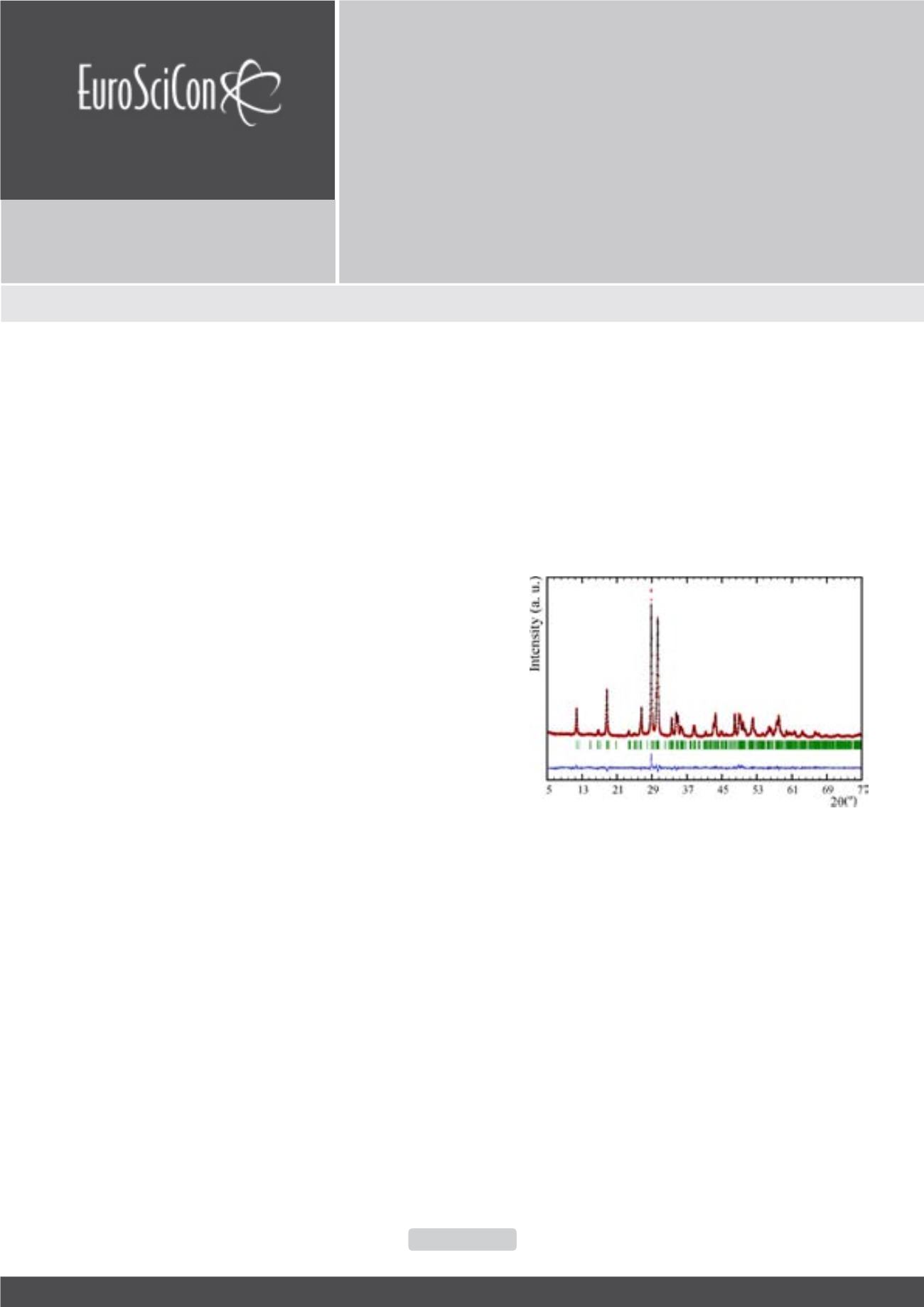
structure with space group of P21/c (Figure 1), as was expected.
The XRD patterns show that fluorination induces a shift in peak
position to lower angles corresponding to an increase in unit cell
sizes as the total anion content increases. The characterization of
these new systems will be reported.
Figure 1:
Rietveld refinement of the cuspidine-related Eu
4
Al
2
O
9
phase (space group
P21/c).
Recent Publications
1. Ghosh S (2015) Thermal barrier ceramic coatings-a
review, in: A.M.A. Mohamed (Ed.), Advanced ceramic
processing, InTech.
2. Zhou X, Xu Z, Fan X, Zhao S, Cao X, He L (2014)
Y4Al2O9 ceramics as a novel thermal barrier coating
material for high-temperature applications. Materials
Letter 134:146-148.
3. Martín-Sedeño MC, Marrero-López D, Losilla ER,
Bruque S, Núñez P, Aranda MAG (2006) Stability
and oxide ion conductivity in rare-earth aluminium
cuspidines. Journal of Solid State Chemistry
179:3445-3455.
FLUORINATION OF CUSPIDINE-RELATED PHASES, LN
4
AL
2
O
9
(LN=SM,
EU, GD, TB)
Aroa Moran-Ruiz
1
, Aritza Wain-Martín, Alodia Orera, María Luisa Sanjuán,
Aitor Larrañaga, Peter R. Slater
and
Maribel Arriortua
University of the Basque Country, Spain
Aroa Moran-Ruiz et al., Struct Chem Crystallogr Commun 2018, Volume 4
DOI: 10.21767/2470-9905-C1-005
















