
Cardiology Insights 2019
March 07-08, 2019
Berlin, Germany
New Horizons in Cardiology
& Cardiologists Education
22
nd
International Conference on
Journal of Heart and Cardiovascular Research
ISSN: 2576-1455
Page 20
Which cells are involved in
cardiomyogenesis in mammalian and
zebrafish heart?
Galina B Belostotskaya
Sechenov Institute of Evolutionary Physiology and Biochemistry, Russia
T
here is no clear evidence on which cells are able
to renew the adult mammalian myocardium.
Studying cardiomyogenesis in the heart of newborn
mammals and adult zebrafish, many researchers have
concluded that new cardiomyocytes (CMs) are formed
by dedifferentiation and division of pre-existing mature
CMs. In addition, it is supposed that mature CMs of
zebrafish are divided throughout life, not only renewing
the myocardium, but also regenerating it after injury. In
turn, it has been shown that mature CMs of mammals
are divided only in the first 5-7 days after birth, and
then permanently lose this ability. Investigating the
phenomenon of intracellular development of resident
cardiac stem cells (CSCs) with the formation of “cell-
in-cell structures” (CICSs), we’ve found that transitory
amplifying cells (TACs), being released after CICSs
opening, are 2 times larger than the original CSCs
(12-13 µm vs. 5-6 µm) and are able to divide and
differentiate. We observed the presence of CICSs
not only in the myocardium of adult mammals, but
also in 18-day-old embryos and the neonatal rats. We
found that CICSs, formed in the embryonic phase, not
only provide TACs for embryonic cardiomyogenesis,
but, opening immediately after birth, release large
numbers of proliferating TACs to support neonatal
cardiomyogenesis. Counting the number ofmitotic cells
and measuring their size showed that only small cells
with D<13 µm are able to divide in the neonatal period.
After that, their proliferation stops, and they transit
from hyperplasia to hypertrophy. We demonstrated that
adult myocardium of Danio rerio also contains CICSs.
Upon opening, they release a large number of TACs, the
dimensions of which are comparable to the dimensions
of the cells that divide inside the myocardium of
newborn mammals. We assume that specifically TACs,
but not mature CMs, form new CMs in mammals and
zebrafish throughout life.
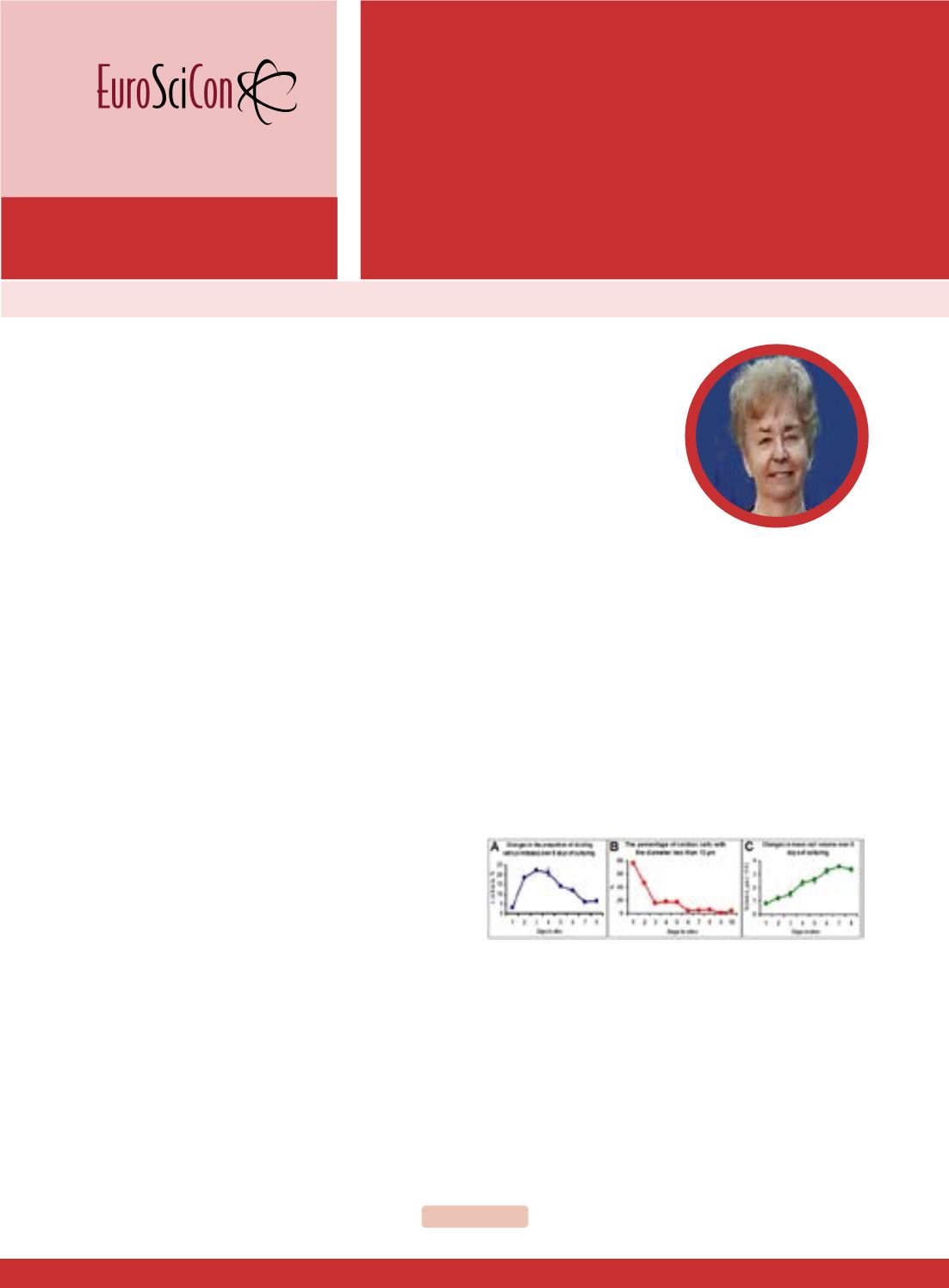
Figure 1
: Growth of myocardial cells obtained fromnewborn rat heart
in vitro. Proportion of dividing cells (A), proportion of cells with D<13
µm (B) and mean cell volume (C).
Recent Publications
1. Belostotskaya G B and Golovanova T A
(2014) Characterization of contracting
cardiomyocyte colonies in the primary culture
of neonatal rat myocardial cells: Amodel of in
vitro cardiomyogenesis. Cell Cycle 13(6):910-
Galina B Belostotskaya, J Heart Cardiovasc Res 2019, Volume 3
DOI: 10.21767/2576-1455-C1-001
















