ISSN : 0976-8505
Der Chemica Sinica
Voltammetric Analysis of ATPase-inhibitor Using Redox Mediator Platform: Application in Biological Fluids
Pakinaz Youssef Khashaba1,2, Hassan Refat Hassan Ali1 and Mohamed Mahmoud El-Wekil1*
1Department of Pharmaceutical Analytical Chemistry, Faculty of Pharmacy, Assiut University, Egypt
2Department of Pharmaceutical Analytical Chemistry, Faculty of Pharmacy, Deraya University, El-Minya, Egypt
Abstract
In this study, the oxidation of rabeprazole sodium (RAB Na+), a widely used anti-ulcer drug, was investigated at a modified poly (bromocresol green)/ pencil graphite electrode (poly (BCRG)/PGE). A disposable, sensitive and selective electrochemical platform is proposed for the determination of RAB Na+ by recording its cyclic voltammetry (CV) and square wave adsorptive voltammetry (SWV) in Britton-Robinson buffer solution at pH of 7.0 using the modified PGE. The poly BCRG/PGE displayed a good electrochemical behaviour with significant enhancement of the peak current compared to the bare PGE. Under experimental conditions, the modified electrode had a linear response range from 10-180 × 10-8 M and 3-330 × 10-7 M for SWV and CV, respectively. The detection limit was found to be 3 × 10-8 M and 1 × 10-7 M for SWV and CV, respectively. These voltammetric methods were successfully applied for the direct determination of RAB Na+ in real samples. The effect of various interfering substances on the RAB Na+ peak current was also investigated.
Keywords
Bromocresol green, Pencil graphite electrode, Rabeprazole sodium, Real samples
Introduction
Peptic ulcers form painful ulcers in the lining of the stomach or in the first part of the small intestine (duodenum) produced by excessive secretion of HCl. These ulcers can cause serious health problems including bleeding, stomach and duodenal perforation or swelling. The newest group discovered to treat peptic ulcers are proton pump inhibitors (PPIs) such as rabeprazole sodium (RAB Na +) that irreversibly inhibit ATPase enzyme [1]. Few methods were used to determine RAB Na+ as spectrophotometry [2,3], spectrofluorometry [4], thin layer chromatography [2] and highresolution liquid chromatography [2,5]. Two electrochemical methods [6,7] have been applied for the determination of RAB Na+ in the bulk drug and pharmaceutical dosage form. Electrochemical techniques have attracted more attention in recent times for the analysis of electro-active compounds with biological, clinical, and environmental application [8-10]. The use of modified pencil electrodes is well documented [11-18]. These electrochemically treated electrodes are compact, inexpensive, easy to prepare and easy to use as working electrodes for both laboratory analysis and on-pot detection analysis. The ability to modify these electrodes in one step is very important. The pencil graphite electrode (PGE) can be applied for analysis of drugs and in trace detection of metal ions [19]. This electrode has a larger active surface area and is therefore capable of detecting low concentrations of the analyte. Polymer-modified electrodes have the advantages of improving electro catalysis, the absence of surface fouling and the prevention of undesirable reactions that compete kinetically with the desired electrode procedure [20-22]. It has been shown that the electrodes modified with polymers show excellent stability, reproducibility and homogeneity [23-25]. Most redox dyes are artificial electron donors [26,27] and are capable of electro-polymerization to generate active redox layers [28].
In this work, bromocresol green was chosen as monomer to obtain poly (BCRG)/PGE by electrochemical polymerization for the first time where the process was simple and rapid. In addition, because of the high hydroxyl (OH) groups in the bromocresol green molecule made the polymer dye to have good concentrations of negatively charged surface functional groups. The modified electrode showed excellent electro-catalytic properties in the determination of RAB Na+ making it suitable for the analytical purpose. Therefore, the present work was carried out for the first time to apply poly (BCRG)/PGE to determine RAB Na+ electrochemically in tablets and biological fluids.
Materials and Methods
Pharmaceutical products
RAB sodium was supplied as a gift from Global Napi, 6th October city, Giza, Egypt. Rabicid® tablets (Sigma, Quesna, El-Menoufia, Egypt) were labeled to contain 40 mg RAB sodium. Domperidone was supplied as a gift from EIPICO, 10th Ramadan city, El-Sharquia, Egypt. Aceclofenac, tinidazole, and clarithromycin were obtained as gifts from NODCAR, El-Giza, Egypt. Doxycycline was supplied as a gift from CID, Assiut, Egypt.
Reagents and solvents
Glacial acetic acid, phosphoric acid, boric acid, potassium ferricyanide, potassium chloride and ascorbic acid were purchased from El Nasr Pharmaceutical Chemicals Co., Egypt. Bromocresol green, uric acid and dopamine were purchased from Sigma-Aldrich, Germany. Double distilled water was used in all the steps.
Instrumentation
For electrochemical measurements a Princeton VersaSTAT MC (VersaSTAT 3, Princeton Applied Research, AMETEK, USA) was used to connect to a three-electrode cell. In all measurements, the reference electrode was Ag / AgCl, KCl the auxiliary electrode was a platinum wire and PGE as a working electrode. The electrical contact with the wire was obtained by welding a wire to the metal part holding the cable in place within the pencil. Unless otherwise indicated, the pen was fixed so that about 3 mm of its length would be immersed in the solution. The measurements were carried out in a 10 ml glass cell containing 6 ml of electrolytic support solution.
The stirring was obtained with a magnetic bar. pH values were measured using a Hanna pH meter (Hanna Instruments Brazil, São Paulo, Brazil). The solutions were ultrasound using a Bransonic ultrasonic cleaner, Branson UL, Eagle Road, Danbury, CT 06813, USA. Surface morphological superficial studies of the modified electrode were performed using scanning electron microscopy (SEM), JEV JSM-5400 LV (Oxford, USA) instrument. A Nicolet 6700 FTIR Advanced Gold Spectrometer, supported with OMNIC software (Thermo Electron Scientific Instruments Corp., WI USA) for data processing.
Preparation of standard solutions
1.0 mM RAB Na+ was prepared by dissolving the appropriate amount of the drug in 20 ml of double distilled water for five minutes to ensure complete solubility of the drug. The volume was completed to 100 ml using double distilled water. Standard work solutions were prepared by further diluting the stock solution with B.R. buffer (pH=7.0).
Sample preparation
The contents of ten tablets were carefully weighed, finely powdered and thoroughly mixed in a mortar. Parts equivalent to about 1.0 mM of the drug were carefully weighed and dissolved in 20 mL of distilled water. The contents were subjected to sonication for about 20 minutes to ensure complete solubility. The excipients were removed by centrifugation at 3000 rpm for 5 minutes. The residue was washed three times with double distilled water. The volume was completed to 100 ml using double distilled water.
Human blood free of drug has been obtained by healthy volunteers. In order to remove serum proteins, acetonitrile (0.75 ml) was added to 1.0 ml of the serum sample and diluted to B.R. (pH 7.0) in a 10 ml volumetric flask and the mixture was centrifuged for 10 min at 5000 rpm. The supernatant was then carefully taken and used for further analysis.
Drug-free urine samples were obtained from healthy volunteers and non-smokers of different age and sex. Samples were stored at -20°C and analyzed the day after collection without further pretreatment. One milliliter of the corresponding urine sample is pipetted into a 50 ml calibrated flask and packed in volume with B.R. buffer (pH=7.0).
Results and Discussion
Deposition of poly (BCRG) films on the surface of PGE and parameters that influence the deposition
Preparation of poly (BCRG)/PGE
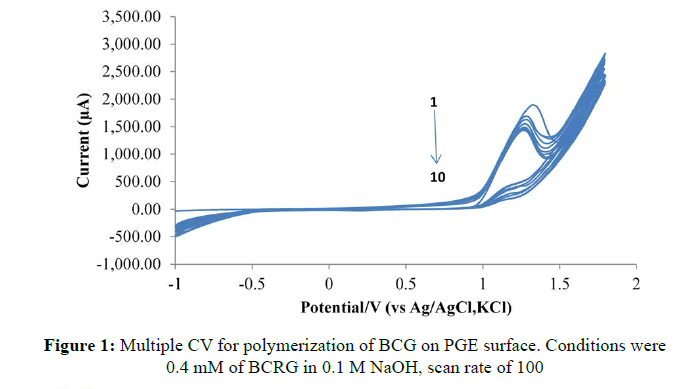
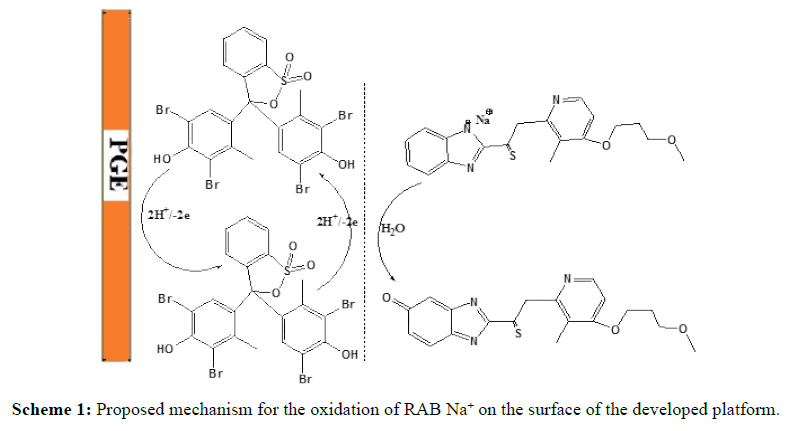
Figure 1 shows the CV of BCRG electro-polymerization on the surface of PGE. The results have shown that the oxidation peak for BCRG is gradually diminished during multiple successive cycles (Scheme 1).
Poly (BCRG) Film Study on PGE
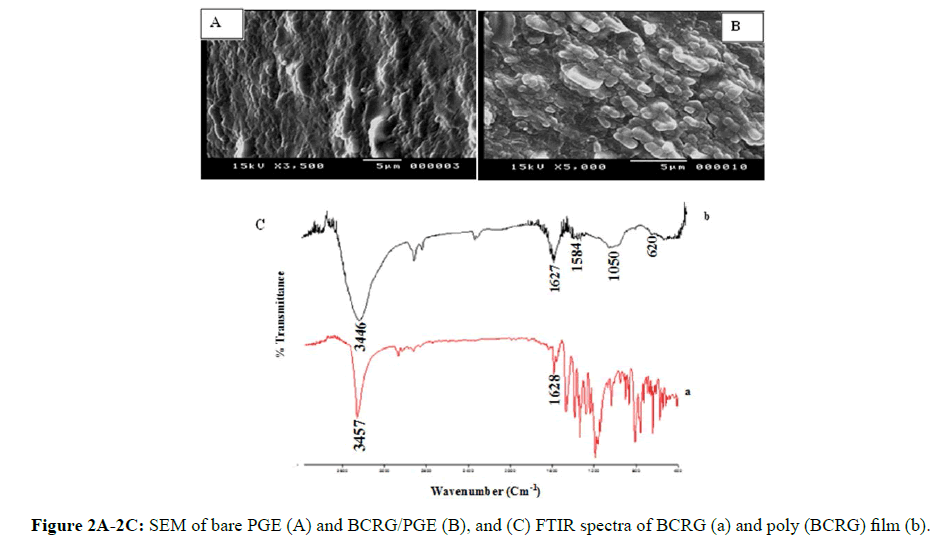
The Electron Scanning Microscope (SEM) was used to study the interfacing morphology of the electrode surfaces. Figures 2A and 2B show the surface morphology of PGE bare and poly (BCRG)/PGE, respectively. Figure 2B shows that the surface of PGE was coated with a film of a uniform scale, indicating that the BCRG film deposited successively on the surface of the electrode and can surprisingly increase the interaction between the modified electrode and the analyte. Figure 2C illustrates the FTIR spectra of the monomer BCG (a) and poly (BCG) (b). The peak at 3446 cm-1 is O-H stretching vibration peak and the peaks at 1584 and 1628 cm-1 are benzene skeleton vibration peaks. As shown in curve b, it can be seen that the peaks of S=O stretching vibration (1050 cm-1), the peaks of C-Br stretching vibration (620 cm-1) and the peaks of benzene skeleton vibration (1520 cm-1).
Factors affecting the deposition of BCRG in PGE
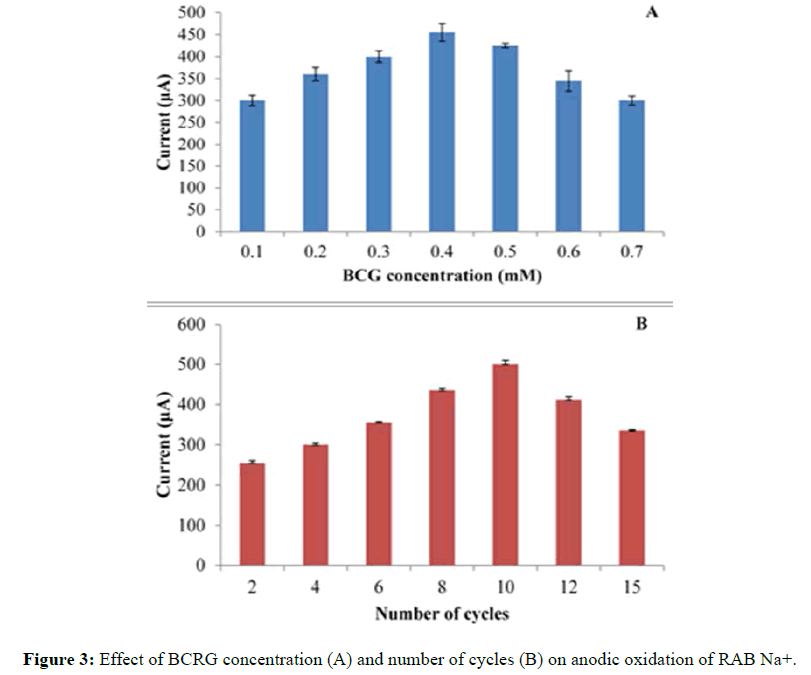
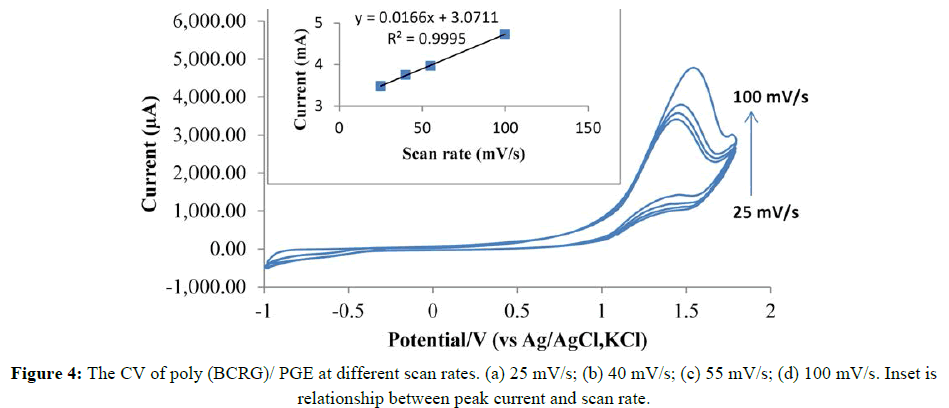
The maximum current of the drug was increased with a growing deposition of BCRG which in turn increased with an increasing concentration of BCRG up to 0.4 mM (Figure 3A). The effect of BCRG polymerization cycles on RAB Na+ oxidation current has been studied. Peak current has been reached to maximum after cycles increased to 10 cycles after that the current decreased (Figure 3B). This may due to blocking the PGE surface by excessive amount of the dye. The effect of scan rate on redox behaviour of the poly (BCRG) film was investigated in the range of 25-100 mVs-1 (Figure 4).
Electrochemical characterization of the poly (BCRG/PGE) using standard potassium ferricyanide
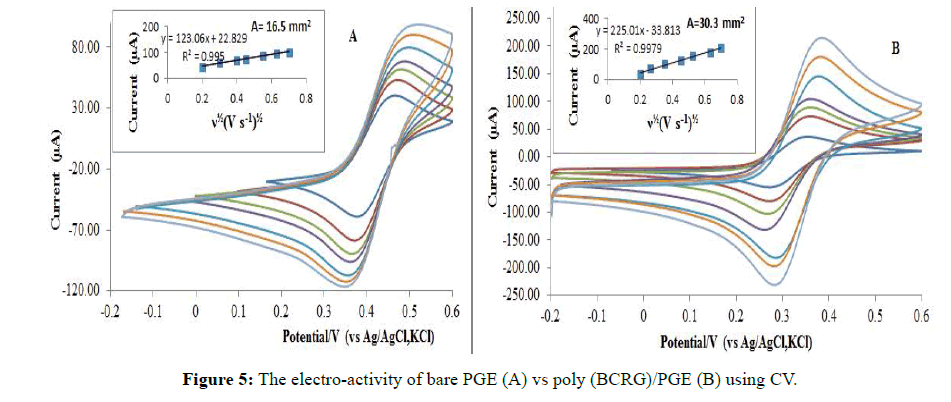
Before the voltammetric analysis, the surface activity of PGE was evaluated before and after modification using cyclic voltammetry (CV). The CV was recorded after immersion of PGE/modified PGE in 1 mM ferricyanide potassium mixed with 0.5 M KCl. Randles -Sevcik equation for a reversible process [29] was used to estimate the effective surface (Aeff.mm2) of the electrodes.
Where D and C° are the diffusion coefficient and ferricyanide mass concentration, respectively. The surface area of the bare PGE was 16.5 mm2 (Figure 5A), while the poly (BCRG)/PGE was 30.3 mm2 (Figure 5B).
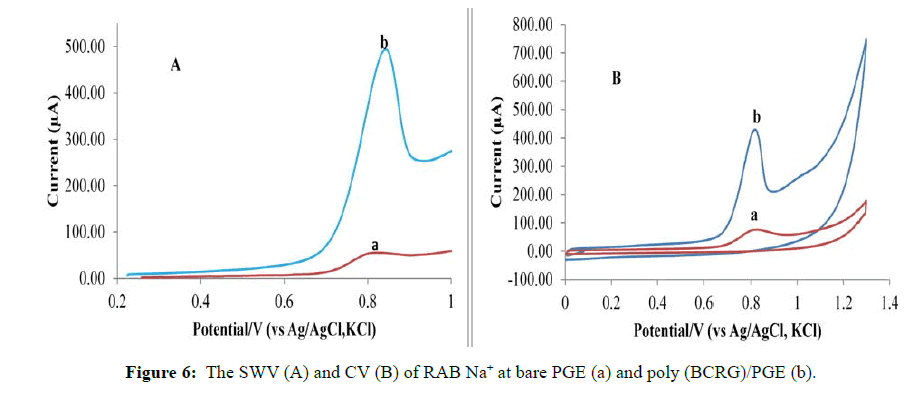
Electrochemical characterization of RAB Na+ on bare electrode against modified electrode
The CV and SWV were presented in (Figures 6A and 6B). The CV of RAB Na+ showed only an irreversible anode peak at +850 mV and no cathodic peak was recorded in reverse scanning.
Analytical parameters involved in the determination of RAB Na+
The effect of pH
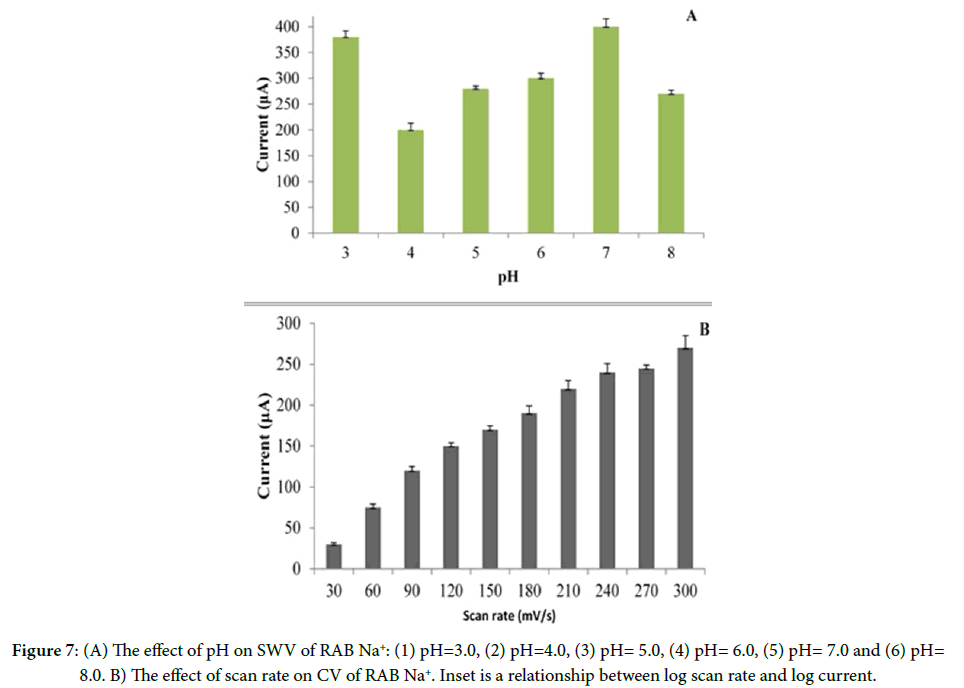
Britton-Robinson buffer compared to other supporting electrolytes produced better results. Therefore, B.R. buffer was chosen as a suitable supporting electrolyte. A well-defined oxidation peak was recorded at pH 7.0. The maximum potential (Epa) was shifted to the less positive side with a pH increase. The optimal result with respect to the sensitivity was obtained at pH 7.0 (Figure 7A).
The effect of the scan rate on CV of RAB Na+
Figure 7B shows the effect of the scan rate (ʋ) in the range of 20-120 mV s-1 on the oxidation peak of RAB Na+. It was found that the logarithm of the peak oxidation current (log I) is linear to the logarithm of the scan speed (log ʋ), with the linear regression equation: log I=0.787 + 1.989 log ʋ. From the slope value, it can be deduced that the oxidation process of RAB Na+ is a controlled diffusion adsorption with a contribution process [30].
Method validation
The optimum conditions for the determination of RAB Na+ using SWV were: adsorption potential=0.2 V, adsorption time=20 seconds, frequency=180 Hz, step potential=20 mV and pulse amplitude=55 mV while CV conditions were adsorption potential=0 V, adsorption time=20 seconds and scan rate=300 mVs-1.
Linearity, LOD and LOQ
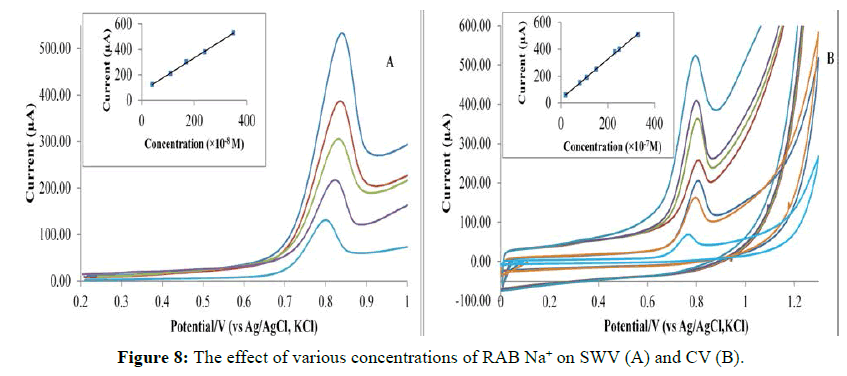
The spontaneous adsorption of RAB Na+ on the surface of modified PGE can be exploited as a high sensitivity possibility for its determination because of the effective accumulation stage before its voltammetric measurement. Figure 8 shows the linear relationship between the concentration and the anodic current. From Table 1, it is evident that CV and SWV methods are sensitive to the determination of RAB Na+ in LOD and LOQ terms.
| Analytical parameters | SWV | CV |
|---|---|---|
| Linearity rangea | 15-180 | 20-330 |
| Correlation coefficient (r) ± SD* | 0.9993±0.02 | 0.9991±0.01 |
| Intercept (a)± SD* | 106.5± 2.1 | 31±0.5 |
| Slope (b)± SD* | 2.1±0.02 | 1.5±0.015 |
| LOQa | 10 | 3 |
| LODa | 3 | 1 |
| aConcentration in SWV expressed as 10-8M while in CV as × 10-7M. *Average of five replicates. |
||
Table 1: The quantitative parameters of the proposed SWV and CV methods.
Accuracy and precision of the proposed platform
The accuracy of the proposed platform has been confirmed by the standard addition method and calculation of recovery percentages. In addition, the results of intra-day and intra-day precision demonstrate the reproducibility of the proposed methods (Table 2).
| Voltammetric method | Standard addition method | Precision | ||||
|---|---|---|---|---|---|---|
| Amount addeda | Found ±SD* | % Recovery ± SD* |
Amount ataken | Inter-day | Intra-day | |
| CV | 50 | 51 | 102± 2 | 80 | 99± 1.5 | 100± 2 |
| 100 | 98 | 98± 2 | 110 | 101±1 | 103±2 | |
| 200 | 197 | 98.5± 1 | 140 | 98± 1 | 101±1.5 | |
| SWV | 50 | 50.5 | 101± 1.5 | 100 | 100± 1.5 | 100± 1 |
| 80 | 81 | 101± 1.5 | 150 | 102± 1 | 101± 1.5 | |
| 120 | 116 | 97± 2 | 180 | 98± 2 | 100± 1 | |
| aConcentration in SWV expressed as 10-8M while in CV as × 10-7 M. *Average of five replicates. |
||||||
Table 2: Analysis of Rabicid® tablets by standard addition method and precision of the proposed methods.
Proposed platform selectivity
The effects of excipients, administered drugs, biological compounds and divalent cations were evaluated (Table 3). It is obvious that the % change in the RAB Na+ signal after the addition of these potential interfering substances has not been significantly altered. This may indicate the selectivity of the method.
| Common excipients | Biological active substances | Co-administered drugs | Divalent metals | ||||
|---|---|---|---|---|---|---|---|
| Amount (1mM) | %signal change |
Amount (1mM) |
%signal change |
Amount (20 µM) | %signal change | Amount (0.3 µM) |
%signal change |
| Starch | 2.5 | Ascorbic acid | 3.5 | Domperidone | 5 | Manganese | 3 |
| Glucose | 3 | Uric acid | 4 | Aceclofenac | 3 | Nickle | 3 |
| Gum acacia | 4 | Dopamine | 2 | Metronidazole | 0.5 | Copper | 9 |
| Lactose | 5 | - | - | Clarithromycin | 1 | Cadmium | 6 |
| Citric acid | 2.5 | - | - | Doxycycline | 4 | Zinc | 4 |
| - | - | - | - | - | - | Chromium | 6 |
Table 3: The effect of potential components on the voltammetric response of RAB Na+.
Real sample application
The proposed methods were applied for the determination of RAB Na+ using the standard addition method in tablets (Table 2), urine samples and serum (Table 4) where the results obtained confirmed that there were no interferences with biological active compounds.
| Amount addeda | Human blood serum | Human urine | ||||||
|---|---|---|---|---|---|---|---|---|
| SWV | CV | SWV | CV | |||||
| Found | % Recovery ± SD* | Found | % Recovery ± SD* | Found | % Recovery ± SD* | Found | % Recovery ± SD* | |
| 50 | 48 | 96±2 | 50.5 | 101±2.5 | 52 | 104±2 | 51 | 102±1.5 |
| 100 | 103 | 103±1.5 | 101 | 101±2 | 102 | 102±4 | 101 | 99±3 |
| 130 | 133 | 102±2 | 128 | 98.5±1.5 | 132 | 101.5±2 | 131 | 101±2 |
| aConcentration in SWV expressed as 10-7M while in CV expressed as µM. *Average of five replicates. |
||||||||
Table 4: Recovery of the proposed methods in human serum and urine.
Modified electrode stability
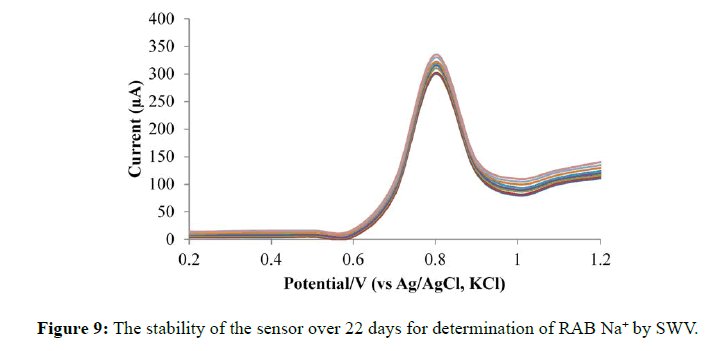
The modified electrode stability built has also been studied in 0.1 M NaOH solution at room temperature and electrode measurements were measured for a period of 26 days. The electrode retained 95% of its original signal for the determination of RAB Na+ for about three weeks. This revealed that there were no surface deterioration for a period of three weeks if stored in NaOH solution (0.1 M) (Figure 9).
Comparison with other reported methods
The newly developed method for determining RAB Na+ was compared with other reported methods. Obviously, the developed methods had shown high sensitivity and applicability with respect to other methods (Table 5).
| Reported methods | Linear range (µg/mL) | LOD (µg/mL) | LOQ (µg/mL) | Reference |
|---|---|---|---|---|
| Spectrophotometry | 10-30 | 0.019 | 0.058 | [2] |
| 5-40 | 0.19 | 0.57 | [3] | |
| Spectrofluorometry | 10-85 | 2.99 | 9.07 | [4] |
| HPTLC | 0.5-2.5 | 0.1 | 0.31 | [2] |
| 4-20 | 0.025 | 0.076 | [2] | |
| HPLC | 0.02-1.5 | ----- | 0.02 | [5] |
| 1-20 | 0.4 | 1 | [6] | |
| Differential pulse voltammetry | ||||
| SWV | 0.06-0.7 | 0.01 | 0.04 | This work |
| CV | 0.76-12.6 | 0.03 | 0.1 | |
Table 5: Comparison between the proposed methods and reported methods.
The proposed mechanism
The proposed oxidation mechanism in Scheme 1 is based on the electrochemical data described and supported by the RAB Na+ structure. The oxidation phase in the ortho position will be favored (by resonance) from stabilizing the radical size. The preferred attack is on the aromatic ring, where the degree of conjugation is greater, making it easier to eliminate an electron. Two electrons were removed, followed by deprotonation to produce a cationic radical, which reacts with water and leads to the formation of quinone species [31].
Conclusion
A modified pencil graphite electrode was successfully fabricated using the bromocresol green (BCRG). Poly (BCRG)/ PGE has good catalytic activity for RAB Na+ oxidation. It could be seen that electrochemical oxidation was greatly improved due to the bromocresol green with respect to the bare electrode. The modified platform showed superior performance in peak current, linear field of operation, detection limit. The sensor was applied for the determination of RAB Na+ in real samples such as pharmaceutical tables, human urine and blood serum samples. The selective determination of RAB Na+ is possible in the presence of potentially interfering species since they do not alter the actual reaction of the drug. The proposed method is simple, precise and does not require any pre-treatment phase such as extracting an analyte from pharmaceutical and biological samples. In addition, the sensor offered minimal interference, good reproducibility and stability. The proposed method is efficient and more sensitive than other methods reported.
References
- Patrick GL (2009) An introduction to Medicinal Chemistry. 4th edn., Oxford, USA, pp: 653-680.
- El-Gindy A, El-Yazby F, Maher MM (2003) Spectrophotometric and chromatographic determination of rabeprazole in presence of its degradation products. J Pharm Biomed Anal31:229-242.
- El-Kommos ME, Khashaba PY, El-Wekil MM (2013) A validated spectrophotometric assay of some proton pump inhibitors using diazotized P-nitroaniline in alkaline medium. Asian J Biomed Pharm Sci3: 31-38.
- El-Kommos ME, Khashaba PY, El-Wekil MM (2014) Validated spectrofluorimetric method for the assay of some proton pump inhibitors using sodium-12-naphthoquinone-4-sulphonate.Inter J Pharm Sci6: 212-219.
- Noubarani M, Keyhanfar V, Motevalian M, Mahmoudian M (2010) Improved HPLC method for determination of four PPIs, omeprazole, pantoprazole, lansoprazole and rabeprazole in human plasma. J Pharm Pharmaceut Sci 13: 1-10.
- Radi A, Abd El-Ghany N, Wahdan T (2004) Voltammetric behaviour of rabeprazole at a glassy carbon electrode and its determination in tablet dosage form. FARMACO 59: 515-518.
- Khashaba PY, Ali HR, El-Wekil MM (2017) Highly sensitive and selective complexation based voltammetric methods for the analysis of rabeprazole sodium in real samples. RSC Adv 7: 3043-3050.
- Malgorzat G, Cecyli W (2014)A new voltammetric strategy for sensitive and selective determination of gallium using cupferron as a complexing agent. J Environ Sci Health 49: 1142-1148.
- Shahryar A, Abbas F, Seyede SM (2010) Ultrasensitive simultaneous quantification of nanomolar level of Cd and Zn by cathodic adsorptive stripping voltammetry in some real samples. Electroanalysis 22: 2884-2888.
- Xuefei G, Woo H, Noe A, Vesselin NS, William RH (2013) Detection of trace zinc by an electrochemical microsensor based on carbon nanotube threads. Electroanalysis 25: 1599-1604.
- Bond AM, Mahon P J, Schiewe J, Vicente-Beckett V (1997) An inexpensive and renewable pencil electrode for use in field-based stripping voltammetry. Anal Chem Acta345: 67-74.
- King D, Friend J, Kariuki J (2010) Measuring vitamin c content of commercial orange juice using a pencil lead electrode. J Chem Ed87:507-509.
- Kariuki JK (2010) An Electrochemical and spectroscopic characterization of pencil graphite electrodes. J Electrochem Soc 159: 747-751.
- Chauhan N, Narang J, Pundir C (2010) Amperometric determination of serum cholesterol with pencil graphite rod.Am J Anal Chem 2:41-46.
- Demetriades D, Economou A, Voulgaropoulos A (2004) A study of pencil-lead bismuth-film electrodes for the determination of trace metals by anodic stripping voltammetry. Anal Chem Acta 519: 167-172.
- Pokpas K, Zbeda S, Jahed N, Mohamed N, Baker PG, et al. (2014) Electrochemically reduced graphene oxide pencil graphite in situ plated bismuth-film electrode for the determination of trace metals by anodic stripping voltammetry. Int J Electrochem Sci 9: 736-759.
- Vestergaard M, Kerman K, Tamiya E (2005) An electrochemical approach for detecting cooper-chelating properties of flavonoids using disposable pencil graphite electrodes: Possible implications in copper-mediated illnesses. Anal Chem Acta 538: 273-281.
- Hartati YW, Topkaya S, Maksum IP, Ozsoz M (2013) Sensitive detection of mitochondrial DNA A3243G tRNALeu mutation via an electrochemical biosensor using meldola’s blue as a hybridization indicator. Advances Anal Chem 3A: 20-27.
- Chandra U, Kumara BE, Gilbert O, Sherigara BS (2011) Cyclic Voltammetric Investigation of Dopamine at Poly-(Gabapentin) modified carbon paste electrode. Int J Electrochem1:1-5.
- Bedioui F, Devynck J, Bied-Charreton C (1995) Immobilization of metalloporphyrins in electropolymerized films: Design and applications. Acc Chem Res28:30-36.
- Nambiar S, Yeow JTW (2011) Conductive polymer-based sensors for biomedical applications. Biosens Bioelectron 26: 1825-1832.
- Podlovchenko BI, Andreev VN (2002) Electrocatalysis on polymer-modified electrode. Russ Chem Rev71: 837-851.
- Liu X, Luo LQ, Ding YP (2012) Simultaneous determination of L-cysteine and L-tyrosine using Au-nanoparticles/poly-eriochrome black T film modified glassy carbon electrode. Bioelectrochemistry 86:38-45.
- Sun W, Wang YH, Zhang YY, Ju XM, Li GJ, et al. (2012) Poly(methylene blue) functionalized graphene modified carbon ionic liquid electrode for the electrochemical detection of dopamine. Anal Chim Acta 751:59-65.
- Periasamy AP, Ho YH, Chen SM (2011) Multiwalled carbon nanotubes dispersed in carminic acid for the development of catalase based biosensor for selective amperometric determination of H2O2 and iodate. Biosens Bioelectron 29: 151-158.
- Ardakani MM, Karimi AA, Mirdehghan SM, Zare MM, Mazidi R (2008) Electrocatalytic determination of hydroxylamine with alizarin red S as a homogenous mediator on the glassy carbon electrode Sens. Actuators B 132: 52-59.
- Park DH, Laivenieks M, Guettler MV, Jain MK, Zeikus JG (1999) Microbial utilization of electrically reduced neutral red as the sole electron donor for Growth and metabolite production. Appl Environ Microbiol65: 2912-2917.
- Karyakin AA, Bobrova OA, Karyakina EE (1995)Electroreduction of NAD+ to enzymatically active NADH at poly(neutral red) modified electrodes. J Electroanal Chem 399: 179-184.
- Bard AJ, Faulkner LR (1980) Electrochemical Methods, Fundamentals and Applications, Wiley, New York.
- Laviron E (1979) General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem 101: 19-28.
- Jorge SMA, Pontinha ADR, Oliveira-Bretta AM (2010) Electrochemical redox behavior of omeprazole using a glassy carbon electrode. Electroanalysis 22: 625-631.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences