ISSN : 2348-9502
American Journal of Ethnomedicine
Vasodilatory Effects of Aqueous Extract from Harungana madagascariensis Stem Bark in Isolated Rat Aorta: The Roles of Endothelium and K+ Channels
1Department of Biological Sciences, Higher Teachers’ Training College, University of Yaounde I, P.O Box 47, Yaounde, Cameroon
2Medical Research Centre, Institute of Medical Research and Medicinal Plants Studies (IMPM), P.O Box 13033, Yaounde, Cameroon
3Department of Animal Biology and Physiology, Faculty of Sciences, University of Yaounde I, P.O Box 812, Yaounde, Cameroon
4Department of Chemistry, Higher Teachers’ Training College, University of Yaounde I, P.O Box 47, Yaounde, Cameroon
- *Corresponding Author:
- Esther Ngo Lemba Tom
Department of Biological Sciences
Higher Teachers’ Training College
University of Yaounde I, Cameroon
Tel: +237-699828838
E-mail: esther_ngotom@yahoo.com
Received Date: October 12, 2018; Accepted Date: October 31, 2018; Published Date: November 05, 2018
Citation: Tom ENL, Mimb JRB, Nyunaї N, Bekono YF, Longo F, et al. (2018) Vasodilatory Effects of Aqueous Extract from Harungana madagascariensis Stem Bark in Isolated Rat Aorta: The Roles of Endothelium and K+ Channels. Am J Ethnomed Vol.5 No.1:8
DOI: 10.21767/2348-9502.10008
Abstract
Background: Hypertension is the most modifiable risk factor for cardiovascular diseases worlwide. It is one of the leading causes for mortality, as it may be asymptomatic but a lot of complications will develop rapidly and leading to death. Prevention and control of hypertension decreases mortality, and heart failure. The aim of this study was to investigate the vascular activity of aqueous extract obtained from stem bark of Harungana madagascariensis (Hypericaceae) and to determine the pharmacological mechanisms of the extract on rat aorta in vitro.
Methods and Findings: The activity of different concentrations of H. madagascariensis aqueous extract (HMAE) was evaluated on contractile responses of isolated aorta to phenylephrine (PE, 1 μM) and potassium chloride (KCl, 60 mM). Then, various pharmacological agents were used to assess the involved vascular mechanisms. The extract (10-3- 8.10-1 mg/mL and 6.10-1-1 mg/mL) induced a concentration dependent relaxation respectively in aortic rings precontracted by PE and KCl. The effect on PE-preconstricted aortic rings was significantly reduced after endothelium removal, whereas it was enhanced on KCl-preconstricted rings. L-NAME, methylene blue and indomethacin as well as tetraethylammonium and barium chloride reduced significantly this vasorelaxation after PE-induced contraction. The vasorelaxant effect of sodium nitroprusside was not modified in the presence of HMAE.
Conclusions: The involvement of NO-cGMP as well as prostacyclin-cAMP pathways contributes to the endothelium-dependent relaxant effects of HMAE after PEinduced contraction. Contribution of Ca2+-activated K+ channels, voltage-activated K+ channels and K+ inward rectifier channels as endothelium-independent mechanisms could also explain its vasorelaxants effects. The observed data suggest that HMAE has potential effects as phytoalternative treatment for hypertension and other cardiovascular diseases.
Keywords
Harungana madagascariensis; Vasorelaxant effect; Endotheliumdependent; Potassium channels; hypertension; rat
Introduction
Non communicable diseases (NCDs) were reported to cause 38 million (68%) of 56 million human deaths worldwide in 2012. The four main NCDs are cardiovascular diseases (CVDs, 46% of all NCDs deaths), cancers (22%), respiratory diseases (10.7%) and diabetes (4%) [1]. Arterial hypertension (AH) is one of the prevalent CVDs [2]. By 2025 the number of hypertensive patients around the world will reach 25% of adults [3]. Uncontrolled AH is considered as a major risk factor for stroke, myocardial infarction, ischemia, blindness and kidney diseases. In Sub-Saharan Africa, AH has been on the rise with reports indicating higher values in urban settings compared to rural settings [4,5]. The development of synthetic drugs for hypertension treatment has increased. Important classes of antihypertensive drugs are sympatholytic drugs, diuretics, Ca2+ channel blockers, angiotensin converting enzyme inhibitors, angiotensin II receptor antagonists, and vasodilators [6]. Antihypertensive drugs influence arterial blood pressure at four effector sites: the resistance vessels, the capacitance vessels, the heart, and the kidney [7]. Because these drugs are effective but also cause many side effects, approximately 80% of the world population uses various herbal medicines because of their low toxicity and better acceptability by the human body [8]. Thus, many vasodilator compounds derived from plants such as resveratrol [9], chlorogenic acid [10] as well as alkaloids, terpenoids and flavonoids have been identified [11].
Harungana madagascariensis Lam. ex Poir belong to the family ‘Hypericaceae’, earlier known as ‘Guttiferae’ and is commonly known as haronga, orange-milk or dragon’s blood tree [12]. It is native to Madagascar, Mauritius and tropical Africa growing on margins of wet forests [13]. Preparations from different parts of H. madagascariensis locally called ‘atondo’ in Ewondo (a tribe of Center Cameroon) have been used for the treatment of a wide spectrum of human diseases. The bark is employed in the treatment of malaria, river blindness, ulcer, asthma, hepatitis, dysmenorrhea, toothache and hypertension. The leaves of H. madagascariensis are used for the treatment of chest pains and urogenital infections. Fruits are used as an abortive [14]. Documented studies indicate isolation of bioactive compounds like anthrones, anthraquinones, xanthones, flavonoids, and essential oils from this plant [15,16]. Scientific validation of the reported medicinal uses indicate that H. madagascariensis posses antidiarrhoeal and antibacterial effects [17]; antioxidant effects [18]; anti-inflammatory, anti-plasmodial, antidiabetic and analgesic activities [19-21]. Recently, we demonstrated that H. madagascariensis exert hypotensive and cardioprotective effects [22,23]. To date, the effect of H. madagascariensis stem barks on the changes of the vascular tone has not been studied yet. Therefore, this study was aimed to investigate for the first time, the effects of stem bark aqueous extract of H. madagascariensis on isolated rat thoracic aortic rings as well as the possible mechanisms involved.
Materials and Methods
Plant material and extraction
Fresh H. madagascariensis stem barks were collected at Essezok, Mbalmayo (Center Region, Cameroon) in June 2016. The identification of the plant was done at the Cameroon National Herbarium, where a voucher sample was deposited under the registration number No. 4224 HNC. Bark pieces were dried under room temperature and powdered with the help of electrical grinder. One kilogram of powder was introduced into 12 L of distilled water and boiled for 20 minutes. The resulting decoction was filtered through Whatman paper No. 3 and further lyophilized. A crude brown extract powder (HM extract, 98.90 g) was obtained, giving a yield of 9.89%.
Animals
Adult male Wistar rats weighing 200-250 g were used. The animals were housed in cages at 22 ± 5°C temperature and given tap water and standard diet ad libitum, while a 12 h on/12 h off light cycle was maintained. All experiments were conducted in accordance with the internationally accepted principles for laboratory animal use and care and with institutional guidelines.
Preparation of aortic rings
Animals were anaesthetized by intraperitoneal injection of ketamine/diazepam (50/10 mg/kg, i.p). Then, the thorax was opened and the thoracic aorta was quickly removed and cleaned from adherent connective tissues and cut into rings (2-3 mm in length). All the dissection procedures were performed with extreme care to protect the endothelium from inadvertent damage. Aortic rings were suspended horizontally between two stainless steel hooks. One of the hooks was fixed to the chamber wall, while the other was attached to an isometric force transducer (50, EMKA Technologies, France). Isometric contractile responses were determined by placing the rings in an organ bath (20 mL) (Organ Bath, 2 channels, EMKA Technologies, France) containing Krebs–Henseleit solution (37°C, pH 7.4) of the following composition (mM): NaCl 118, KCl 4.65, CaCl2 2.52, MgSO4 1.64, KH2PO4 1.18, NaHCO3 24.9 and glucose 12 and gassed with oxygen. Tissues were then maintained under 2 g initial tension and allowed to equilibrate for 1 h during which bath solution was replaced every 15 min [24]. In some aortic rings, the endothelium was removed by gently rubbing the inner surface with a rough steel needle, and used as the denuded endothelium aorta. In all experimental groups, after equilibration, the presence of a functional endothelium was assessed on the basis of more than 70% relaxation evoked by the endothelium–dependent dilator acetylcholine (ACh, 10-6 M) in aorta rings precontracted with phenylephrine (10-6 M).
Effects of H. madagascariensis extract on phenylephrine or potassium chloride-induced aortic contraction
For the study of the vasodilatory effects of H. madagascariensis, the thoracic aorta rings isolated from rats were contracted by using phenylephrine (10-6 M, PE) or potassium chloride (60 mM, KCl) in two separate experiments. When the vasoconstriction curves for the rings reached the plateau phase of maximum tension, the extract was cummulatively added in the organ bath at the respective concentrations of 10-3, 4 × 10-3, 8 × 10-3, 10-2, 4 × 10-2, 8 × 10-2, 10-1, 4 × 10-1, 6 × 10-1 and 8 × 10-1 mg/mL for the contraction induced by PE and 6 × 10-1, 6 × 5 × 10-1, 7 × 10-1, 7 × 5 × 10-1, 8 × 10-1 and 1 mg/mL for the contraction induced by KCl. The tensions were recorded and the vasorelaxant effect of H. madagascariensis was calculated as a percentage of the relaxation in response to PE and KCl on the aortic rings.
Effects of H. madagascariensis on endotheliumintact or endothelium-denuded aortic rings precontracted with phenylephrine or potassium chloride
To examine the role of endothelium on the vasorelaxing effect of H. madagascariensis, the vasodilatory effect of H. madagascariensis on constriction induced by PE or KCl in rat thoracic aortas was also evaluated for both intact endothelium rings and denuded endothelium rings. To confirm that the endothelial layer had been removed, we selected the rings with a maximum relaxation induced by acetylcholine less than 30% after phenylephrine (10-6 M)-induced contraction in denuded vessels.
Studies of endothelium-related vasorelaxing mechanisms of H. madagascariensis
To determine the endothelium-underlying mechanisms, the role of nitric oxide (NO) and cyclic guanosine monophosphate (cGMP) was determined using the nitric oxide synthase inhibitor, Nω- nitro-L-arginine methyl ester (L-NAME) and the guanylate cyclase inhibitor, methylene blue (MB) respectively. Endothelium-intact rings were incubated with L-NAME (10-4 M), or MB (10-5 M) for 30 min before the addition of PE. The extract of H. madagascariensis was then added cumulatively and the concentration-response curves were constructed for the inhibitory responses.
Effect of H. madagascariensis on endotheliumintact aortic rings pre-incubated with various K+ channel blockers
In order to know the role of K+channels on the extractinduced relaxation, intact aortic rings were pre-incubated with tetraethylammonium chloride (TEA, 10-5 M), a blocker of big Ca2+-activated K+ channels and barium chloride (BaCl2, 10-5 M), an inward-rectifier K+ channel inhibitor for 30 min before PE (10-6 M)-induce contraction. Then the extract (10-3, 4.10-3, 8.10-3, 10-2, 4.10-2, 8.10-2, 10-1, 4.10-1, 6.10-1 and 8.10-1 mg/mL) was added and cumulative concentration-response curves were obtained.
Effect of H. madagascariensis on endotheliumintact aortic rings pre-incubated with indomethacin
To determine if prostanoids were involved in the relaxant effect of H. madagascariensis (10-3-8.10-1 mg/mL) endotheliumintact aortic rings were incubated with indomethacin (10-5 M), a non-selective cyclooxygenase inhibitor for 30 min prior to precontraction with PE (10-6 M). Then the extract (10-3-8.10-1 mg/ mL) was added and cumulative concentration-response curve was obtained.
Effect of H. madagascariensis on sodium nitroprusside induced vasorelaxation
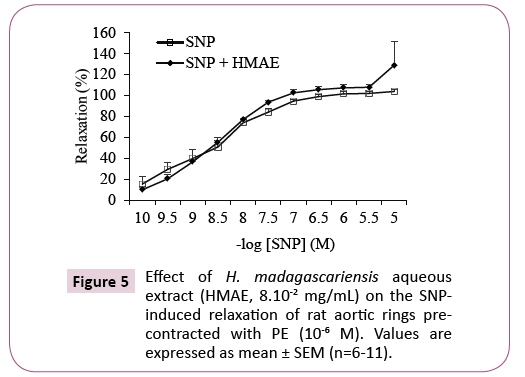
In order to investigate if the extract could affect the response of the vascular smooth muscle to NO through the soluble guanylyl cyclase (sGC) signaling pathway, we evaluated the vasorelaxant response of the blood vessels in the presence of a NO donor sodium nitroprusside (SNP), which activates the sGC pathway alone, and after incubating with the extract (EC50, 8.10-2 mg/mL) and PE induced pre-contraction.
Chemicals and drugs
All chemicals were of analytical grade commercially available. Phenylephrine hydrochloride (PE), acetylcholine chloride (ACh), Nw-nitro-L-arginine methyl ester (L-NAME), sodium nitroprusside (SNP), methylene Blue (BM), tetraethylammonium chloride (TEA), Baryum chloride (BaCl2), were obtained from Sigma-Aldrich (Germany). The stock solutions of these chemicals were prepared by dissolving with distilled water, at concentrations of 10-2 M. The solutions were prepared fresh on the day of experiments.
Data analysis
All data are expressed as mean ± SEM. Two pharmacological parameters were determined from the concentration–response curves (analyzed by nonlinear regression (curve fit) using GraphPad Prism (Version 5.0, GraphPad Software, San Diego, CA, USA): The EC50, the negative logarithm of EC50 (concentration required to achieve a half-maximal response), and Emax expressed as the percentage (%) of the maximal effect of the test substance). Statistical comparisons were made using two-way ANOVA followed by the Bonferroni’s test. P values less than 0.05 were considered to be statistically significant.
Results
Effect of H. madagascariensis on rat aorta rings precontracted with phenylephrine or potassium chloride
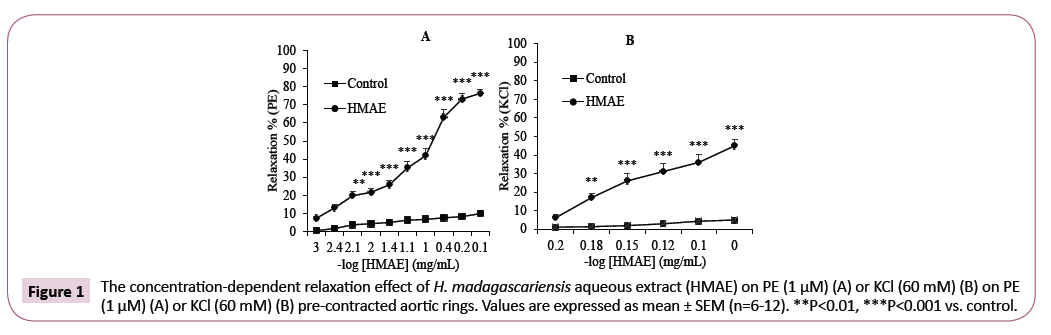
The H. madagascariensis extract induced concentrationdependent relaxation in endothelium-intact aortic rings precontracted by PE or KCl. The maximum relaxant effect (Emax) was 76.3 ± 2.2% (EC50=8.10-2 mg/mL) and 45.0 ± 3.3% (EC50=6.7 × 10-1 mg/mL) at the concentrations of the extract of 8.10-1 mg/ mL and 1 mg/mL respectively (Figure 1A and 1B). In this study, we found the optimal concentration to generate a complete dose-response for H. madagascariensis by studying the results of several tests after PE or KCl-induced contraction.
Role of endothelium in H. madagascariensisinduced relaxation on aortic rings precontracted with PE or KCl
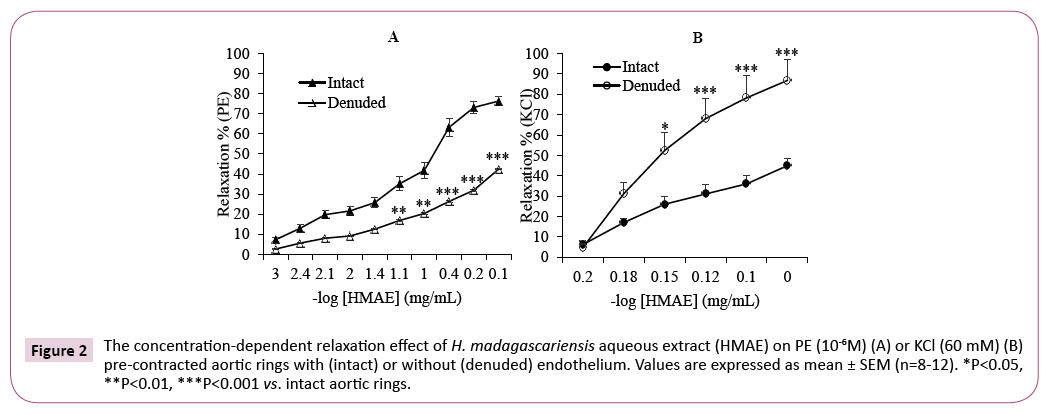
In this series of experiments, we examined the vasorelaxing effect of H. madagascariensis on vascular smooth muscle using endothelium-denuded rat aortic rings. As illustrated in Figure 2, H. madagascariensis induced relaxation in a concentrationdependent manner for endothelium-intact and denuded vessels. However, removal of the endothelium cause dual effect in blood vessels when contracted with PE or KCl: A significant decrease (P<0.001) in the vasorelaxation produced by H. madagascariensis was observed, with an Emax of 42.4 ± 2.2% in denuded vessels vs. 76.3 ± 2.2% in intact rings when contracted with PE (Figure 2A). Surprisingly, the removal of endothelium enhanced the relaxation induced by H. madagascariensis when rings were pre-contracted with KCl. In this case, the Emax was 87.0 ± 10.2% in denuded rings vs. 45.0 ± 3.3% in intact rings (P<0.001) (Figure 2B).
Figure 2: The concentration-dependent relaxation effect of H. madagascariensis aqueous extract (HMAE) on PE (10-6M) (A) or KCl (60 mM) (B) pre-contracted aortic rings with (intact) or without (denuded) endothelium. Values are expressed as mean ± SEM (n=8-12). *P<0.05, **P<0.01, ***P<0.001 vs. intact aortic rings.
Participation of the NO-cGMP pathway on the vasorelaxant effect of H. madagascariensis
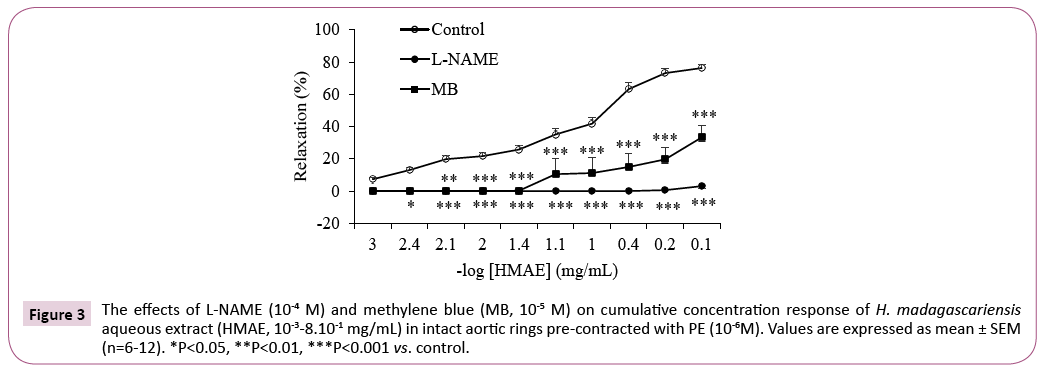
Pre-treatment of intact aortic rings with L-NAME (10-4 M, a non selective NOS inhibitor) or methylene blue (MB, 10-5 M, a guanylate cyclase inhibitor) produced a significant change (P<0.001) of the response and vasorelaxation was markedly inhibited (Figure 3). In the presence of L-NAME or MB the Emax was 3.0 ± 1.2% and 33.3 ± 7.3%, respectively vs. 76.3 ± 2.2% in the control aortic rings.
Figure 3: The effects of L-NAME (10-4 M) and methylene blue (MB, 10-5 M) on cumulative concentration response of H. madagascariensis aqueous extract (HMAE, 10-3-8.10-1 mg/mL) in intact aortic rings pre-contracted with PE (10-6M). Values are expressed as mean ± SEM (n=6-12). *P<0.05, **P<0.01, ***P<0.001 vs. control.
Role of cyclooxygenase on H. madagascriensis induced relaxation on aortic rings with endothelium
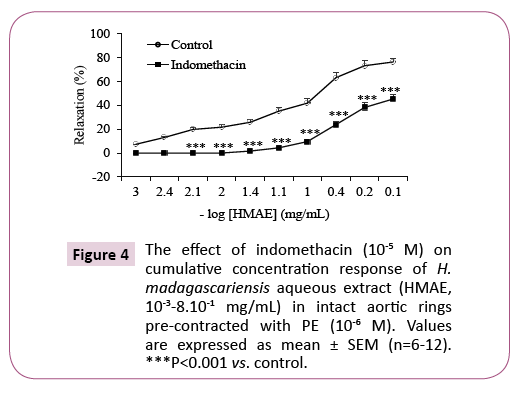
In the presence of indomethacin (10-5 M, a non-selective cyclooxygenase inhibitor) the relaxation induced by the aqueous extract of H. madagascariensis was significantly decreased on aortic rings precontracted with PE. The Emax decreased from 76.3 ± 2.2% to 45.3 ± 6.0% respectively in absence and in the presence of the indomethacin (Figure 4). This represents an inhibition of 31% (P <0.001).
Effect of H. madagascariensis on the concentration-relaxant effect of SNP
As shown in Figure 5, the relaxation responses of the vessels to SNP were similar in the absence as well as in the presence of the extract.
Role of potassium channels in H. madagascariensis-induced relaxation of aortic rings
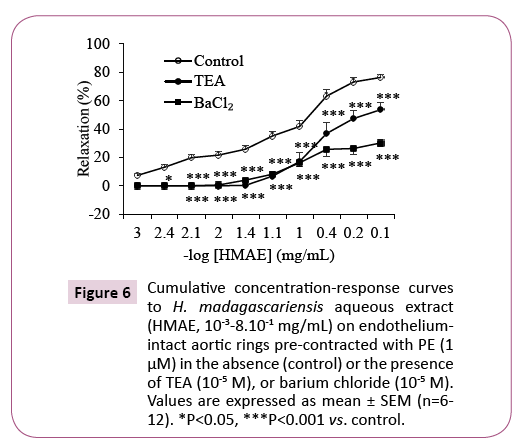
The vasorelaxant effect of the aqueous extract of H. madagascariensis was significantly (P<0.001) inhibited both in the presence of tetraethylammonium (TEA, 10-5 M), a nonselective calcium-dependent wide K+ channel blocker, and in the presence of barium chloride (BaCl2, 10-5 M), an inward-rectifier K+ channel inhibitor. The Emax decreased from 76.34 ± 2.2% in the absence of antagonists to 53.61 ± 5.2% and 30.18 ± 2.8% respectively in the presence of TEA and BaCl2 (Figure 6).
Figure 6: Cumulative concentration-response curves to H. madagascariensis aqueous extract (HMAE, 10-3-8.10-1 mg/mL) on endotheliumintact aortic rings pre-contracted with PE (1 μM) in the absence (control) or the presence of TEA (10-5 M), or barium chloride (10-5 M). Values are expressed as mean ± SEM (n=6-12). *P<0.05, ***P<0.001 vs. control.
Discussion
The results of the present study showed that H. madagascariensis stem bark extract elicits concentration-dependent relaxation on endothelium-intact aortic rings pre-contracted by PE or KCl. This observation demonstrates that H. madagascariensis aqueous extract (HMAE) possess a direct vasodilatory effect on vascular smooth muscle. Added to the fact that HMAE has recently been shown to exert hypotensive effect [22] and considering the increasing interest in traditional medicines, H. madagascariensis may represent a potential candidate for the treatment of hypertension.
The mechanisms of contraction involved in response of arterial smooth muscle cells to PE and KCl are different. PE is an α1 adrenoceptor agonist which induced contraction through various calcium entry mechanisms or channels such as receptor-operated calcium channels (ROCCs), capacitative calcium entry (CCE) by the activation of store operated calcium channels (SOCCs), reversal mode of sodium calcium exchangers (NCX), and non-capacitative calcium entry (NCCE) via the activation of diacyl glycerol (DAG) lipase [25,26]. KCL induced contraction mainly by Ca2+ influx upon the cell membrane depolarization which activates voltagedependent L-type Ca2+ channels (VDCCs) [27]. The vasorelaxing effect of HMAE was more potent in aortic rings preconstricted with PE than those preconstricted with KCl, suggesting that the blockade of ROCCs may be an important factor in the relaxation induced by HMAE.
The endothelium plays a critical role in determining vasotone, and receptors located on the endothelial surface are primary targets for initiating vasodilatory effects [28,29]. In this study, we observed a dual effect depending on the contracting agent used after endothelium removal in aortic rings. In the presence of KCl, the vasorelaxant effect of HMAE was enhanced in denuded rings compared to intact rings while in denuded rings preconstricted with PE, the relaxant effect of HMAE was significantly reduced. It seems that in the presence of KCl after endothelium removal, endothelium derived-contracting factors (EDCFs) which counteracts vasodilation in response to vasodilator agents are eliminated, thus resulting in an enhancement of vasodilation due to the absence of EDCFs or endothelium, endothelium removal activated some putative factor to enhance relaxation in reponse to HMAE. These findings are similar with those reported in isolated rat thoracic rings precontracted with norepinephrine, where lidocaine induced-relaxation was significantly enhanced by endothelial removal [30]. And also with those reported in rat mesenteric arteries constricted with methoxamine, where endothelium removal augments the vasodilation of some agents such as isoprenaline, sodium nitroprusside and 8-bromo-cGMP [31].
It is well known that the endothelium releases vasorelaxant agents such as endothelium derived-relaxing factors (EDRF) and prostacyclin (prostaglandin I2) which induces vasorelaxation of vascular smooth muscle cells and modulates vascular tone [32]. The major EDRF is nitric oxide (NO). It is induced by nitric oxide synthase (NOS) and leads to the enhancement of vasorelaxation through NO-cGMP pathway via guanylate cyclase activation [33]. In endothelial cells, the calcium-calmodulin complex stimulates NO synthase (NOS), which later activates NO formation from L-Arginine. NO then enters the smooth muscle cells and stimulates guanylate cyclase, which increases intracellular cyclic guanosine monophosphate (cGMP). The increase in intracellular cGMP then stimulate cGMP-dependent protein kinases leading to a decrease in the calcium concentrations in the smooth muscle cells, which causes its relaxation [34]. In this study, to better understand the effect of HMAE in denuded ring after PE contraction, we further used NOS and guanylate cyclase inhibitors. The vasorelaxant effect of HMAE was abolished by NOS inhibitor, Nw nitro-Larginine methyl ester (L-NAME) and significantly attenuated by guanylate cyclase inhibitor, methylene blue. Prostacyclin produced by cyclooxygenase-1 from arachidonic acid increases cAMP, which leads to vascular smooth muscle relaxation [35]. These results show that the cyclooxygenase inhibitor, indomethacin, also inhibited the vasorelaxation induced by HMAE in isolated rat thoracic aorta rings. Therefore, we suggest that the vasorelaxant activity of HMAE may be exerted mainly via the NO-cGMP pathway and partially via the prostacyclin-cAMP pathway.
Sodium nitroprusside (SNP), an endothelium-independent relaxant agent is considered as one of the NO donors most widely studied [36]. Its effect is attributed to its direct action on the vascular smooth muscle (VSM). HMAE did not have any effect on the relaxation elicited by sodium nitroprusside (SNP) after PEinduced contraction, suggesting that endothelium-dependent mechanisms are more likely involved in the vasorelaxant effect of HMAE.
The change in K+ channel activity is an important mechanism of vasoconstriction and vasodilation as it changes the activity of voltage-dependent Ca2+ channels [37]. To investigate the possibility that the vasorelaxant effects of HMAE are mediated via K+ channels, TEA (a Ca2+-activated K+ channel blocker and non-specific voltage-activated K+ channel blocker) and BaCl2 (inwardly rectifying K+ channel blocker) were used [38,39]. The vasorelaxant effect of HMAE was significantly attenuated by pre-treatment with TEA and BaCl2, suggesting that the relaxant response of HMAE was involved in the role of K+ channel opening of Ca2+-activated K+ channels, voltage-activated K+ channel and K+ inward rectifier channels in vascular smooth muscle cells.
Conclusions
The aim of this study was to evaluate the vascular effects of HMAE and to characterise the mechanisms in isolated rat aorta. HMAE induced vasorelaxation in rat aortic rings precontracted by either PE or KCl. The involvement of NO-cGMP as well as prostacyclincAMP pathways contributes to its endothelium-dependent relaxant effects after PE-induced contraction. Ca2+-activated K+ channels, voltage-activated K+ channels and K+ inward rectifier channels were also involved in the vasorelaxant effect of HMAE. The observed data suggest that HMAE has potential effects on the regulation of the hypertension. More studies are required to determine its anti-hypertensive effects in hypertensive animals and to elucidate the signaling mechanisms. In addition, further studies are needed to characterize the active compound responsible for its cardiovascular properties.
Conflicts of Interest
The authors declare no conflict of interest.
Funding
This study was financed by the International Foundation For Sciences (IFS) under the Research Grant N° F/5882-1.
References
- World Health Organization (2014) Global status report on noncommunicable diseases (2014) Geneva.
- Veeramani C, Al-Numair KS, Chandramohan G, Alsaif MA, Alhamdan AA, et al. (2012) Antihypertensive effect of Melothria maderaspatana leaf fractions on DOCA-salt-induced hypertensive rats and identification of compounds by GC-MS analysis. J Nat Med 66: 302-310.
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, et al. (2005) Global burden of hypertension: analysis of worldwide data. Lancet 365: 217-223.
- Mufunda J, Mebrahtu G, Usman A, Nyarango P, Kosia A, et al. (2006) The prevalence of hypertension and its relationship with obesity: results from a national blood pressure survey in Eritrea. J Hum Hypertens 20: 59-65.
- Malhotra R, Puone T, Hoyo C, Hughes G, Ostbye T (2008) Prevalence and awareness of hypertension in an urban township of South Africa: compelling need for action. Ethnicity and Disease 18: 401-402.
- Chandra KS, Ramesh G (2013) The fourth-generation calcium channel blocker: cilnidipine. Indian Heart J 65 : 691-695.
- Gerber JG, Nies AS (1990) Antihypertensive agents and the drugs therapy of hypertension. In: Goodman and Gilman’s The Pharmacological Basis of Therapeutics (8thetn), New York: Pergamon Press-Macmillan, pp: 784-785.
- Tabassum N, Ahmad F (2011) Role of natural herbs in the treatment of hypertension. Pharmacogn Rev 5: 30-40.
- Novakovic A, Bukarica L, Gojkovic M, Peric D, Nezic B, et al. (2006) The mechanism of endothelium-independent relaxation induced by the wine polyphenol resveratrol in human internal mammary artery. J Pharmacol Sci 101: 85-90.
- Tom ENL, Girard-Thernier C, Demougeot C (2016) The Janus face of chlorogenic acid on vascular reactivity: A study on rat isolated vessels. Phytomed 23: 1037-1042.
- Luna-Vazquez FJ, Ibarra-Alvarado C, Rojas-Molina A, Rojas-Molina I, Zavala-Sánchez MA, et al. (2013) Vasodilator compounds derived from plants and their mechanisms of action. Molecules 18: 5814-5857.
- Burkill HM (1985) The useful plants of west tropical Africa. (2ndedn), Families AD. Royal Botanic Gardens, Kew, UK, pp: 960.
- Orwa CA, Mutua A, Kindt R, Jamnadass R, Anthony S, et al. (2009) Agroforestree Database: a tree reference and selection guide version 4.0. Available : https://www.worldagroforestry.org/sites/treedbs/treedatabases.asp. Assessed 20 September 2018.
- Maikere-Fanniyo R, Van Puyvelde L, Mutwewingabo A, Habiyaremye FX (1989) Study of Rwandese medicinal plants used in the treatment of diarrhoea. J Ethnopharmacol 26 : 101-109.
- Kouam SF, Khan SN, Krohn K, Ngadjui BT, Kapche DF, et al. (2005) Prenylated anthranoid antioxidants from the stem bark of Harungana madagascariensis. J Nat Prod 66: 1174-1179.
- Kouam SF, Yapna DB, Krohn K, Ngadjui BT, Ngoupayo J, et al. (2007) Antimicrobial prenylated anthraacene derivatives from the leaves of Harungana madagascariensis. J Nat Prod 70: 600-603.
- Mba JR, Weyepe FCL, Mokale ALK, Tchamgoue AD, Tchokouaha LRY, et al. (2017) Antidiarrhoeal, antibacterial and toxicological evaluation of Harungana madagascariensis. Sch Acad J Biosci 5: 230-239.
- Oboh G, Akomolafe TL, Adefagha SA, Adetuyi AO (2010) Antioxidant and modulatory effect of ethanolic extract of Madagascar harungana (Harungana madagascariensis) bark on cyclaphosphamide induced neurotoxicity in drugs. J Food Drug Anal 18: 171-179.
- Nwodo OFC (1989) Antibiotic and anti-inflammatory analgesic activities of Harungana madagascariensis stem bark. Int J Crude Drug Res 27: 137-140.
- Lenta NB, Ngouela S, Boyom FF, Tantangmo F, Tchouya FGR (2007) Anti-plasmodial activity of some constituents of the root bark of Harungana madagascariensis Lam. (Hypericaceae). Chem Pharmacol Bull 55: 464-447.
- Mangambu M (2014) Contribution à l’étude phytochimique de quelques plantes médicinales antidiabétiques de la ville de Bukavu et ses environs (Sud-Kivu, R. D. Congo). J Appl Biosci 75: 6211-6220.
- Tom ENL, Nankia FD, Mezui C, Nyunai N, Bekono YF, et al. (2018) Mechanisms of the hypotensive action of Harungana madagascariensis (Hypericaceae) stem bark aqueous extract in rats. Int J Curr Adv Res 7: 10580-10584.
- Tom ENL, Nankia FD, Nyunai N, Girard-Thernier C, Demougeot C, et al. (2018) Myocardial potency of aqueous extract of Harungana madagascariensis stem bark against isoproterenol-induced myocardial damage in rats. Univ J Pharm Res 3: 17-24.
- Demougeot C, Prigent-Tessier A, Marie C, Berthelot A (2005) Arginase inhibition reduces endothelial dysfunction and blood pressure rising in spontaneously hypertensive rats. J Hypertens 23: 971-978.
- Jackson WF (2000) Ion channels and vascular tone. Hypertension 35: 173-178.
- Lee CH, Poburko D, Sahota P, Sandhu J, Ruehlmann DO, et al. (2001) The mechanism of phenylephrine-mediated [Ca(2+)](i) oscillations underlying tonic contraction in the rabbit inferior vena cava. J Physiol 534: 641-650.
- Furchgott RF, Zawadzki JV (1980) The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373-376.
- Busse R, Trogisch G, Bassenge E (1985) The role of endothelium in the control of vascular tone. Basic Res Cardiol 80: 475-490.
- Dayang FB, Nur Sa’adah AR, Shafreena SA, Satirah Z (2018) The vasorelaxant effect of Canarium odontophyllum Miq. (Dabai) extract in rat thoracic aorta. Egypt J Basic Appl Sci 5: 75-79.
- Arsyad A, Dobson GP (2016) Lidocaine relaxation in isolated rat aortic rings is enhanced by endothelial removal: possble role of Kv, KATP channels and A2a receptor crosstalk. BMC Anesthesiol: 16: 121.
- Iwatani Y, Kosugi K, Isobe-Oku S, Atagi S, Kitamura Y, et al. (2008) Endothelium removal augments endothelium-independent vasodilatation in rat mesenteric vascular bed. British J Pharmacol 154: 32-40.
- Moncada S, Palmer RMJ, Higgs EA (1991) Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Reviews 43: 109-142.
- Hussain MB, MacAllister RJ, Hobbs AJ (2001) Reciprocal regulation of cGMP-mediated vasorelaxation by soluble and particulate guanylate cyclases. Am J Physiol-Heart Circulat Physiol 280: H1151-H1159.
- Stankevicius E, Kevelaitis E, Vainorius E, Simonses U (2003) Role of nitric oxide and other endothelium-derived factors. Medicina (Kaunas) 39: 333-341.
- Shaul PW, Kinane B, Farrar MA, Buja LM, Magness RR (1991) Prostacyclin production and mediation of adenylate cyclase activity in the pulmonary artery: alterations after prolonged hypoxia in the rat. J Clin Invest 88: 447-455.
- Bonaventura D, Lunardi CN, Rodrigues GJ, Ma´rio A. Neto MA, et al. (2008) A novel mechanism of vascular relaxation induced by sodium nitroprusside in the isolated rat aorta. Nitric Oxide 18: 287-295.
- Brayden JE (1996) Potassium channels in vascular smooth muscle. Clin Exp Pharmacol Physiol 23: 1069-1076.
- Mathie A, Wooltorton JR, Watkins CS (1998) Voltage-activated potassium channels in mammalian neurons and their block by novel pharmacological agents. Gen Pharmacol 30:13-24.
- Novakovic A, Bukarica LG, Kanjuh V, Heinle H (2006) Potassium channels-mediated vasorelaxation of rat aorta induced by resveratrol. Basic Clin Pharmacol Toxicol 99: 360-364.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences