Use of the Brain-Gauge Somatosensory Assessment for Monitoring Recovery from Concussion: A Case Study
King DA1,2* Hume PA1,3 and Tommerdahl M4
1Sports Performance Research Institute New Zealand, Auckland University of Technology, Auckland, New Zealand
2School of Science and Technology, University of New England, Armidale, Australia
3National Institute of Stroke and Applied Neuroscience, Auckland University of Technology, Auckland, New Zealand
4Department of Biomedical Engineering, University of Northern Carolina, Chapel Hill, United States of America
- *Corresponding Author:
- King DA
School of Science and Technology
University of New England, Armidale
NSW, Australia
Tel: 22 034 1580
E-mail: dking@aut.ac.nz
Received Date: January 05, 2018 Accepted Date: January 17, 2018 Published Date: January 30, 2018
Citation: King DA, Hume PA, Tommerdahl M (2016) Use of the Brain-Gauge Somatosensory Assessment for Monitoring Recovery from Concussion: A Case Study. J Physiother Res. Vol.2 No.1:3.
Copyright: ©2018 King DA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objectives: To determine if Brain Gauge somatosensory metrics would adequately reflect an individual’s recovery.
Design: Prospective case study of Ms ‘K’.
Methods: The Brain Gauge somatosensory assessment system was used to show, progress towards recovery from concussion. The battery of tactile tasks was delivered over a 20-minute test period and sensory perceptual metrics (reaction time, amplitude discrimination, temporal order judgment with and without conditioning, and duration discrimination) were obtained. The metrics obtained at post-concussion days 18, 25 and 48 post-injury, were compared to normative values obtained from previously published studies.
Results: Ms ‘K’ recorded 20 sympto Ms with a severity of 77 and the SAC was 24. There were decreases in reaction time, fatigue, amplitude discrimination sequential score, temporal order judgment and the duration discrimination across all three assessments. The temporal order judgment connectivity score increased across all three assessments to be >30% of the temporal order judgment. Temporal order judgment was much higher than normative values for Ms ‘K’ initially but returned to normative values over the time-frame studied. Ms ‘K’s’ timing perception (i.e., duration discrimination) showed notable improvement over the time course examined in this case study.
Conclusion: The multi-parametric approach of cortical metrics was sensitive to the degree of recovery and the diversity of symptom that Ms ‘K’ sustained from her head injury. The Brain Gauge somatosensory metrics enabled rapid visual identification of where Ms ‘K’ was in her recovery from a concussive injury, therefore was a useful tool to help monitor physiological recovery from concussion.
Keywords
Mild traumatic brain injury; Plasticity; Reaction time; Amplitude discrimination; Speed; Accuracy; Connectivity; Temporal order judgment
Key Points
For a minority of people, concussions can take longer than 10 days to recover.
• Use of the symptom indices with the PCS of the SCAT3 can provide a measure of the intensity of the symptoms reported.
• Utilizing a somatosensory system can provide access to a diagnostic system for overall cortical health by enabling access to a somatotopic organization for evoking cortical-cortical interactions.
• The potential for the Brain Gauge to be utilized as a tool to individually assess the recovery of concussions needs to be researched further.
Introduction
Recovery from concussion, or mild Traumatic Brain Injury (mTBI), is difficult to diagnose and often difficult to track. Should a person receive a second concussion, while still recovering from a concussion, the injury can be much more serious, if not fatal [1,2]. As such, during the period between concussion, and full recovery, it is critical that the person not become reinjured. In the initial period, concussions can have adverse effects on cognitive function, balance and have a diverse number, and severity of symptoms [3,4]. There is an increasing body of evidence reporting [5-8] that balance and cognitive deficits, and the symptoms of concussion will return to normal within 10 days for the majority of the population [9]. However, for a sizeable minority of people, this recovery can take longer than 10 days to clinically recover from a concussion, before they are able to return to their normal activities and sporting environments [9-12]. More recently it has been reported that, although people may have clinically recovered from a concussion (i.e., no signs or symptoms), some may not have physiologically recovered (e.g., cerebral blood flow, cortical excitability) [13]. The period of physiological recovery may outlast clinical recovery time, but the duration of this is, as yet unknown [13]. In a study on Cerebral Blood Flow (CBF) post-concussion, it was reported that CBF abnormalities were resolved by 30 days post-injury [14,15]. Studies on Electro Encephalo Gram (EEG) and Event Related Potential (ERP) in concussion have reported persistent effects up to 45 days post-injury [16-19]. In conducting Trans Cranial Magnetic Stimulation (TMS), it was found that there were cortical hypo-excitability persisting beyond the time of clinical recovery, approximately 28 days, and increased inhibition in the motor system that persisted beyond 9 months post-injury [20,21].
Aim
The aim of this case study was to describe the clinical and physiological recovery from concussion utilizing Brain Gauge somatosensory metrics for a case study.
Methodology
The patient assessed throughout this case study is identified as Ms ‘K’ a 50-yr. old female health professional. The use of her information was discussed with her after the last assessment was completed and Ms ‘K’ provided informed consent as to the nature of the study and what details were included. A copy of the manuscript was provided to Ms ‘K’ prior to submission to gain her approval for submission.
Patient clinical management and initial assessment with SCAT3 and BESS
Ms ‘K’ fell off a stationary pushbike and hit her head on the side of a ceramic pot. Sustaining a cut to her ear she was assessed at the local emergency department and treated for this injury (day-0 injury) and provided with two days off work. Ms ‘K’ returned to work and could function in her role during day-3 post injury. Upon returning to work on day-4 she felt unwell and was unable to concentrate. She was referred for a head injury clinical assessment on 4th day. On 4th day, Ms K completed the Sports Concussion Assessment Tool 3rd edition (SCAT3). In addition to the symptom evaluation scales of the SCAT3, symptom indices were applied to the symptom score and severity [22]. The symptoms indices were the Global Severity Index to provide an overall summary measure of the symptoms on a scale from 0 to 6, and the Positive Symptom Distress Index as a measure of the intensity of the symptoms the player reported on a scale from 0 to 6. Ms ‘K’ was medically diagnosed with a concussive injury and was given a return-to-activity program consisting of a further seven days off work, incorporating two days of cognitive rest and 4 days of graduated increase in activities with the aim to return to work in a reduced capacity a week later. Ms ‘K’ was reassessed by her General Practitioner on day-11 and given a further week off work as she did not feel capable of returning to full work duties in the hospital. Upon returning to work on day-18, Ms ‘K’ reported that she felt ‘spacey’ and had difficulty concentrating with her tasks. Ms ‘K’ also reported that her dyslexia appeared to be worse than previously. Her line manager, and Ms ‘K’, approached the lead author (DK) for assistance in developing a graduated return-to-work program. Options were discussed with Ms ‘K’ and her manager, and Ms ‘K’ agreed to a modified return-to-work program and to undergo the Brain Gauge somatosensory assessment as part of this process. Ms ‘K’ underwent three Brain Gauge assessments (post-concussion day 18 [D-18], day 25 [D-25] and day 48 [D-48]) and her return-to-work program was modified to assist with improvements in her recovery.
Sport Concussion Assessment Tool version 3 (SCAT3)
Developed by the Concussion in Sport group, the Sport Concussion Assessment Tool version 3rd (SCAT3) for assessing sports-related concussion [23]. It is the most recent validated tool combining aspects of previous assessment tools into a standardized process. The SCAT3 was utilized to assess, and document the symptoms of concussion, co-modifiers and to conduct a cognitive assessment of Ms ‘K’. There are three sections of the SCAT3 that were utilized for Ms ‘K’. The participant’s background, the test domains for symptom evaluation and the cognitive screening components. The immediate or on-field assessment was not utilized as this was not a field based assessment. The participant’s background provides questions relating to age, gender, hand dominance, history of concussions and recovery; medical imaging for head injury; headaches or migraines; learning disability, dyslexia, attention deficit hyperactive disorder/attention deficit disorder (ADHD/ADD); depression, anxiety or other psychiatric disorder; any familial history of any of these disorders or problems and if they are on any regular medications. In the test domain for symptom evaluation there are two components. The first is to identify the total number of symptoms (0-22; higher score=more symptoms) that are occurring and, following this, to identify the symptom severity (0-132; higher score=more severe symptoms) of those symptoms. The second test domain is the Standardized Assessment of Concussion (SAC) (0-30; lower score=worse cognitive performance), and the modified Balance Error Scoring System (mBESS) comprising of three stances on a hard floor (0-30; higher score=lower number of errors). The timed tandem-gait measure was not completed as part of the SCAT3 assessment. The cognitive assessment in the SAC comprises four components: orientation (0-5), immediate memory (0-15), concentration (digits backwards and months in reverse order, 0-5) and delayed recall (0-5). To evaluate the SCAT3 components the following guidelines for concussion assessment were utilized [24].
Symptom evaluation
• Score (range 1-22): 3 or more symptoms from baseline.
• Severity (range 1-132): score of 11 or more.
Cognitive assessment
• Orientation (range 0-5): 1 less than baseline.
• Immediate memory (range 0-15): 12 or less.
• Concentration (range 1-5): 3 or less for numbers reversed.
• Delayed recall (range 0-5): 3 or less.
• SAC (range 0-30): combined score of 27 or less.
• Modified balance scoring system (range 0-30): more than 3 errors in double and/or tandem stance from baseline.
In addition to the symptom evaluation scales, symptom indices were applied to the symptom score and severity as part of the analysis [22]. The symptoms indices were the Global Severity Index to provide an overall summary measure of the symptoms on a scale from 0 to 6, and the Positive Symptom Distress Index as a measure of the intensity of the symptoms reported on a scale from 0 to 6 [22].
Brain gauge somatosensory assessment
The ‘Brain Gauge’ two-digit vibrio-tactile stimulation hand held device (Brain Gauge. Cortical Metrics, Chapel Hill, NC, USA www.corticalmetrics.com) is the same shape and size to a standard computer mouse (Figure 1).
Sitting at a computer and when logged into the Cortical metrics programmer, the patient was guided through a series of tests. The two cylindrical probes (5 mm in diameter) on the top of the mouse provided a vibration stimulus in the flutter range (25-50 Hz) on the patients second (index, D2) and third (middle, D3) fingers of their non-dominant hand. The custom software running on the laptop computer advised the patient of what actions to take. Ms ‘K’ responded by pressing D2 (left mouse button for digit 2) and D3 (right mouse button for digit 3) with her dominant hand according to instructions of each measurement protocol. Each measurement consisted of a battery of five sequential protocols which lasted approximately 30 minutes. The protocols were similar to previous studies and all amplitude values were measured zero-to-peak [25-29].
Brain gauge test battery
The vibrotactile testing battery consisted of nine tasks. Prior to each task, Ms ‘K’ had to correctly respond to three consecutive practice trials to proceed, in order to confirm that she understood the instructions. Feedback was given during practice trials but not during task trials. In all tasks, stimulus delivery was pseudo-randomized between D2 and D3. All data recorded were visually inspected prior to analysis. In each of the assessments, a simple tracking procedure utilizing a twoalternative forced choice paradigm was undertaken. The tracking procedures for each of the protocols queried Ms ‘K’ as to which of the two stimuli were larger (Amplitude Discrimination [AD]), which of the two stimuli came first (Temporal Order Judgment [TOJ]), or which of the two stimuli lasted longer (Duration Discrimination [DD]). Visual cueing was provided via the computer and practice trials were performed before each test to ensure Ms ‘K’ was familiar with the test. No performance feedback or knowledge of the results was provided during data acquisition.
Brain gauge variables
Brain Gauge normalized scores for speed, accuracy, timing perception, TOJ, connectivity, plasticity and the overall composite (“Cortical Metric”) are described, along with the normative ranges in Table 1.
| Variable & Range | Description of Variable |
|---|---|
| Speed | Computed from reaction time (RT) and variability (RTVar) on the RT tests. Speed is dependent on white matter integrity and frontal-parietal pathways. Disruption of white matter integrity occurs with a number of conditions such as traumatic insult (TBI), MS and some neurodegenerative conditions. High variability has been linked to micro-lesions in the white matter. When performance degrades between the first and second reaction time tests, the Fatigue score will be poor (low value). |
| Reaction Time | |
| 150-200 ms | |
| Reaction Time Variability | |
| 0-20 (or 10% of RT) | |
| Accuracy | Reliant on functional integrity in the parietal lobe and comprises the averaged Amplitude Discrimination (AD) scores (AD sequential (ADseq) and AD simultaneous (ADsimult)). This metric reflects one’s ability to accurately determine which of two stimuli is larger in size (amplitude). Accuracy is reliant on functional integrity in the parietal lobe. Lower numbers are better but both AD values should be similar. Systemic hyperactivity in the cerebral cortex, or an imbalance in excitation/inhibition, can cause a large divergence between the ADseq and ADsimult values. When ADsimult is much greater than ADseq, there are likely problems with lower than normal inhibition or greater than normal activity (hyper-responsively). This can cause a low Plasticity score, which is often observed in chronic pain patients (e.g., migraine), some patients with neurodegenerative problems and patients with traumatic insult, particularly to the parietal lobe. Two stimuli are delivered in each of the AD tests: the stimuli are given one after the other in ADseq while both are delivered at the same time in ADsimult. |
| Amplitude Discrimination Sequential | |
| 20-70 microns | |
| Amplitude Discrimination Simultaneous | |
| 20-70 microns | |
| Temporal Order Judgment | Metric associated with the “when” pathway (frontal-striatal). The measure (in ms) is the smallest time difference between two stimuli such that one can still identify which finger received the first stimulus. Higher than normal values for TOJ are consistent with autism, migraine, non-headache chronic pain, some neurodegenerative disorders, and traumatic insult to the frontal-striatal area. |
| TOJ | |
| 15-35 ms | |
| Time Perception | Metric associated with the duration discrimination (DD) task and the “how long” pathway (cortical-cerebellar). The measure (in ms) is the smallest duration difference between two stimuli that one can perceive. Damage to the cerebellar lobe or the pathway to it results in timing perception getting worse (longer). Higher than normal values for timing perception have been found to be consistent with migraine and non-headache pain as well as traumatic insult to the cerebellum. |
| Duration Discrimination | |
| 30-80 ms | |
| Connectivity | Measure of how well groups of brain cells are communicating with each other. This metric is determined by comparing the scores of the temporal order judgment task (TOJ) and the temporal judgment task carrier (TOJc). TOJc delivers the same stimuli as the TOJ task but with a concurrent conditioning stimulus in order to create an illusion. In the presence of this illusion (or confound), TOJ should be ~30% worse. If the TOJc score is not greater (worse) than TOJ, the connectivity score will be low. |
| Temporal Order Judgment Connectivity | |
| TOJc>30% TOJ | |
| Plasticity | Measure of how well your brain is integrating, processing, and adapting to information from its external environment. States of hyper-excitation (such as can be caused by low GABA levels) lead to poor plasticity scores. Plasticity is a computed metric and a weighted average of lateral inhibition, adaptation and temporal- intensity integration metrics. A comparison of ADseq with ADsimult yields information about lateral inhibition: how well the brain discriminates between two points. ADsimult should not be more than ~50% higher than ADseq. Low GABA can be responsible for low lateral inhibition. A measure of the impact that single site adaptation (SSA) has on amplitude discrimination yields an adaptation metric. The delivered adapting stimulus should be illusory and cause SSA to be ~30% worse than the ADsimult score. A measure of timing perception in the presence of intensity confounds yields a temporal-intensity integration score. Timing perception with confound (DDc) should be ~30% worse than timing perception without the illusion (DD). |
| Lateral inhibition ADsimult>50% ADseq | |
| Adaption | |
| SSA<30% ADsimult | |
| Temporal intensity integration | |
| DDc<30% DD | |
| Cortical Metric | A holistic representation of brain health. It takes the information collected from every available test and computes an “at a glance” view of total brain health. The Cortical Metric is represented as a plot. |
| 0-100% |
Table 1: Description of Brain Gauge variables and normative ranges.
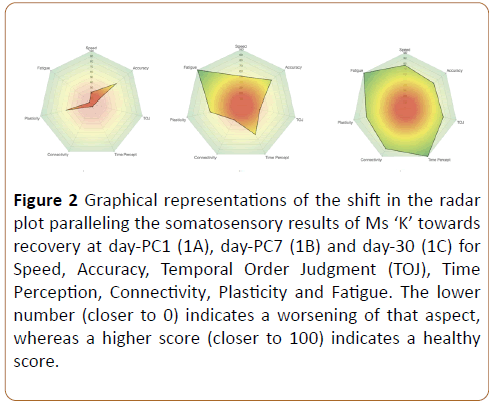
In each case, the score was normalized by comparing the respective measure to the average values previously reported. The normative database was generated by obtaining observations from 448 healthy controls (age range 18-50) that took the same tests in the same manner as the individual described in this case study and a previous concussion study For example, connectivity is a comparison of TOJ to TOJ carrier [26-31]. Plasticity is a comparison of sequential to simultaneous amplitude discrimination and the overall Cortical Metric is the normalized average of all scores. These scores have been implemented as commercialized standards in the Brain Gauge system that is distributed by Cortical Metrics and since they are normalized, can be plotted on a radar plot (Figure 2).
Figure 2: Graphical representations of the shift in the radar plot paralleling the somatosensory results of Ms ‘K’ towards recovery at day-PC1 (1A), day-PC7 (1B) and day-30 (1C) for Speed, Accuracy, Temporal Order Judgment (TOJ), Time Perception, Connectivity, Plasticity and Fatigue. The lower number (closer to 0) indicates a worsening of that aspect, whereas a higher score (closer to 100) indicates a healthy score.
Reaction time
A supra-threshold stimulus (frequency 25 Hz, amplitude 300 μm, duration 40 ms) was delivered on D2 or D3. Ms ‘K’ was asked to respond, as quickly as possible, when they felt the stimulus. 20 trials were delivered with an inter-trial interval of 3 s; 20 trials). The reaction time task was delivered at the beginning and end of each test session.
Amplitude discrimination threshold: Simultaneous vs. Sequential
The AD tasks have been previously described [32,33]. In the simultaneous AD task, Ms ‘K’ was asked to choose which of the two simultaneously delivered stimuli had the higher amplitude (25 Hz; 500 ms; standard stimulus amplitude: 200 μm; initial comparison stimulus amplitude: 400 μm). A 1-up/1-down tracking paradigm (comparison stimulus amplitude was decreased for a correct answer and increased for a wrong answer) was used for the first 10 trials and a 2 up/1 down (two correct answers were necessary for a reduction in comparison stimulus amplitude) was used for the remainder of the task (an inter-trial interval of 5 s; 20 trials). After the simultaneous amplitude discrimination trials, the task was repeated with the same stimulus parameters and the same tracking paradigm, but the two stimuli were delivered 500 ms apart to establish the sequential amplitude discrimination. The order of the stimuli was presented in a pseudo-random manner.
Temporal order judgment (With and without carrier)
In the TOJ task, two single vibrotactile pulses (40 ms, 25 Hz, 200 m) were delivered on D2 and D3 separated temporally by a starting inter-trial interval of 150 ms (the first pulse was assigned pseudo randomly) within a 1-s interval. Ms ‘K’ was asked to respond to the digit that received the first pulse. The TOJ thresholds were taken as the mean of the inter-trial interval of the final five trials. In one condition, there was no concurrent stimulation and in the second condition, a 25 Hz concurrent carrier (20 m) stimulus was delivered throughout each 1-s trial interval.
Duration discrimination
For the DD task, sequential stimuli were delivered to D2 and D3 for 20 trials (initial stimulus parameters: 750 ms test, 500 ms standard, 300 mm, 25 Hz, 25 ms step size). Discrimination capacity was assessed using a two-alternative forced choice tracking protocol in a manner similar to that described for amplitude discrimination capacity. The duration of the test stimulus was always greater than that of the standard stimulus, but the location of the stimulus of longer duration was randomly selected on a trial-by-trial basis. Ms ‘K’ was asked to determine which of the two digits received the longer stimulus duration. The difference between the duration of the test and standard amplitudes was adjusted based on her response; correct responses resulted in shortening the test duration in subsequent trials while incorrect responses resulted in increasing the test duration in subsequent trials (total of 20 trials).
Plasticity
Plasticity, or the ability of the cortex to adapt, is computed by comparison of the results of the sequential and simultaneous AD tasks. In healthy controls, the outcomes of these two tests are typically very similar [34]. Delivery of two stimuli at the same time to the two digits maximally tasks lateral inhibition whereas this is not the case when the same two stimuli are delivered at different times. If there is below normal inhibition, or over-excitation, as is often the case with concussed individuals, then the ability to discriminate between two adjacent and simultaneously delivered stimuli will be compromised (i.e., lateral inhibition will be compromised). Such compromise will negatively impact the plasticity score.
Statistical analysis
All the results of the Brain Gauge were downloaded onto a Microsoft Excel 2016 spreadsheet and analysed with SPSS (IBM Corp, Released 2017. IBM SPSS Statistics for Windows, Version 24.0 Armonk, NY: IBM Corp). Components of the SCAT3 were analysed using a Friedman repeated measures ANOVA on ranks. If significant differences were observed a post-hoc analysis was undertaken by a Wilcoxon signed-rank test. A one-sample chi-squared (χ2) test was used to determine whether the results for D-18 were significantly different from the subsequent scores recorded. Results were considered significant at p<0.05.
Results
SCAT3 with global severity index and positive symptoms distress index
The results of the SCAT3 (Table 2) identified that Ms ‘K’ had some co-modifiers such as migraines, dyslexia and a familial history of the same. On D-4 Ms ‘K’ recorded a symptom score of 20 (GSI 3.5), symptom severity of 77 (PSDI 3.9) and a SAC of 24. Ms ‘K’ scored 10 on the mBESS at the post-injury assessment. Ms ‘K’s symptoms decreased at D-18 (χ2(1)=12.0; p=0.0005; z=-3.1; p=0.0018) and D-25 (χ2(1)=9.0; p=0.0027; z=-2.7; p=0.0065) when compared with D-4.
| SCAT3 Assessment | ||||||
|---|---|---|---|---|---|---|
| Co-Modifiers | Yes/No | |||||
| Previous concussion | No | |||||
| Previous hospitalization / Medical imaging for head injury | No | |||||
| Diagnosed headaches / Migraines | Yes | |||||
| Diagnosed ADD/ADHD; Learning Disability / Dyslexia | Yes | |||||
| Diagnosed Depression; Anxiety; Other Psychiatric Disorder | No | |||||
| Has family history of any of above (Dyslexia, Migraines) | Yes | |||||
| Currently on any medications | No | |||||
| Symptom Evaluation | Range | D-4 Score | D-18 Score | D-25 Score | D-48 score | Signs of Concussion |
| Symptom Score | 0-22 | 20*bc | 14*ac | 10*ab | 2 | 5 or more symptoms |
| General Severity Index | 0.0-6.0 | 3.5 | 1.6 | 0.8 | 0.1 | |
| Symptom Severity | 0-132 | 77*bc | 36*ac | 17*ab | 2 | Combined score>11 |
| Positive Severity Distress Index | 0.0-6.0 | 3.9 | 2.6 | 1.7 | 1 | |
| Cognitive Assessment | Score | |||||
| Orientation | 0-5 | 5 | 5 | 5 | 5 | ≤ 4 |
| Immediate Memory | 0-15 | 14 | 15 | 15 | 15 | ≤ 12 |
| Concentration | 0-5 | 2* | 4 | 5 | 5 | ≤ 3 numbers |
| Delayed Recall | 0-5 | 3* | 5 | 5 | 5 | ≤ 3 |
| Standardised Assessment of Concussion | 0-30 | 24* | 29 | 30 | 30 | ≤ 27 |
| Modified Balance Error Scoring System | 0-30 | 10* | 30 | 30 | 30 | ≤ 3 errors |
Table 2: Modified SCAT3 results with Global Severity Index And Positive Symptoms Distress Index for Ms ‘K’ on day-4 and the last testing session (D-48) following a head injury from a stationary pushbike.
Speed
There were decreases in the reaction time assessment 1 from D-18 (450.2 ms) compared with D-25 (χ2(1)=15.8; p=0.0001) and D-48 (χ2(1)=48.1; p<0.0001) (Table 3).
| D-18 | D-25 | D-48 | |||||
|---|---|---|---|---|---|---|---|
| Measure | Range | Actual | Diff | Actual | Diff | Actual | Diff |
| Reaction Time1 | 150-200 ms | 450.2bce | 250.2 | 338.4ace | 138.4 | 264.8ab | 64.8 |
| Reaction Time2 | 150-200 ms | 325.8bcd | 125.8 | 246.8ad | 46.8 | 257.0a | 57 |
| Reaction Time Variability1 | 0-20 | 65.9bc | 45 | 39.3a | 33.8 | 38.5a | 26.5 |
| Reaction Time Variability2 | 0-20 | 33.4bc | 32.6 | 12.6a | 24.7 | 9.4a | 25.7 |
| Amplitude Discrimination Sequential | 20-70 microns | 80.0b | 10 | 32.0ac | In range | 68.0b | In range |
| Amplitude Discrimination Simultaneous | 20-70 microns | 88 | 88 | 108.0c | 38 | 76.0b | 6 |
| Temporal Order Judgment (TOJ) | 15-35 ms | 108.9bc | 78.9 | 49.6a | 19.6 | 37.4a | 7.4 |
| Temporal Order Judgment Connectivity | >30% TOJ | 109.0b | 0.10% | 53.2ac | 7.30% | 92.7b | 147.90% |
| Duration Discrimination | 30-80 ms | 275.0bc | 195 | 70.0ac | In range | 45.0ab | In range |
Table 3: Brain Gauge somatosensory results for Ms ‘K’ at D-18, D-25 and D-48 post-injury actual scores, and differences from normative range of results.
This was similar for reaction time variability assessment 1 with decreases from D-18 (65.9 ms) compared with D-25 (χ2(1)=6.7; p=0.0095) and D-48 (χ2(1)=7.2; p=0.0073). The reaction time assessment 1 decreased from D-18 (325.8 ms) compared with D-25 (χ2(1)=10.9; p=0.0010) and D-48 (χ2(1)=8.1; p=0.0044).
Fatigue
There were differences in the reaction times between the first and second assessments at D-18 (χ2(1)=19.9; p<0.0001) and D-25 (χ2(1)=8.98; p=0.0027) but not D-48 (χ2(1)=0.12; p=0.7328) indicating a decrease in the effects of fatigue over the duration of the assessments. The reaction time variability decreased between the first and second assessments at D-18 (65.9 vs. 33.4; χ2(1)=10.6; p=0.0011), D-25 (39.3 vs. 12.6; χ2(1)=13.7; p=0.0002) and PC-3 (38.5 vs. 9.4; χ2(1)=17.7; p<0.0001).
Accuracy
The AD sequential score decreased from D-18 to D-25 (χ2(1)=20.6; p<0.0001) to be within the normative range of 20 to 70 microns, but the AD simultaneous score increased from D-18 when compared with D-25 (χ2(1)=2.0; p=0.1531). On D-48, the AD simultaneous score decreased when compared to D-25 (χ2(1)=5.6; p=0.0183).
Temporal order judgment
The TOJ score decreased from D-18 (108.9) when compared with D-25 (χ2(1)=22.2; p<0.0001) and D-48 (χ2(1)=34.9; p<0.0001). TOJ connectivity score increased from D-18 (108.9 ms vs. 109.0 ms; 0.1%) over the three assessments to be >30% of the TOJ (37.4 ms vs. 92.7 ms; 147.9%) at D-48 (χ2(1)=23.5; p<0.0001).
Time perception
The DD score decreased from D-18 (275 ms) when compared with D-25 (χ2(1)=121.8; p<0.0001) and D-48 (χ2(1)=165.3; p<0.0001) to 45 ms.
Discussion
This case study reports on the recovery of Ms ‘K’ from a concussive injury after having been medically cleared to return to full work activities from her GP. With the symptoms returning the decision was made to further evaluate Ms ‘K’. We employed a somatosensory based device to obtain neurosensory assessments of Ms ‘K’ during recovery from her head injury. At D-18 post injury, the results of the Brain Gauge tests identified that Ms ‘K’ was outside of the normative ranges on six of the eight scores recorded on the radar plot. Although her accuracy and plasticity were towards the normative range, the rest of the measurements were outside the normative ranges and her Cortical Metric was 35.7%. Having the visual graph enabled Ms ‘K’ to see where she was at in relationship to the normative ranges. Following these results, a plan was established to assist Ms ‘K’ to recover. This included limited cognitive tasks such as watching screens (computer, television, cell phones) and driving after work longer than 30 minutes. As her primary employment meant using computers to document her care with patients she managed in the emergency department, computer use was limited using a buddy-nursing approach. A space was provided for Ms ‘K’ to have time out in a quiet environment away from the workplace as she required. At D-25, upon re-testing, the results of the Brain Gauge identified that Ms ‘K’ had improved but was still outside of the normative ranges on two of the eight scores recorded. Her fatigue had reduced, Cortical Metric had increased (75.8%) and her speed and accuracy had increased. Ms ‘K’ also reported that her dyslexia had improved following the week of reduced activities and she felt better able to return to work in the ED knowing that she was able to utilize the quiet environment.
Speed
Ms ‘K’ demonstrated an improvement in all the metrics collected. Reaction time and reaction time variability both demonstrated consistent improvement with time postconcussion. Reports on parallels in neurological status and reaction time performance date back to 1868 and this has been well established that both reaction time and reaction time variability are compromised with impaired neurological status [35-37]. Ms ‘K’s’ reaction time and reaction time variability decreased with recovery and led to an improvement in the speed metric. More specifically, reaction time has been demonstrated to be impacted by concussion [38-51], and reaction time variability has been correlated with attention and the inability to focus or attend to activities. The difference between the first and second reaction time and reaction time variability’s can be used to compute a measure of mental fatigue and in the case of Ms ‘K’, this demonstrated improvement post-concussion [41].
Accuracy
Comparison of the sequential vs. simultaneous amplitude discrimination (AD) tasks provides unique insight into the process of lateral inhibition. Both accuracy and plasticity improved time post-concussion for Ms ‘K’. This reflected both an improvement in the AD scores relative to normative values and the relationship between those scores. The accuracy metric is computed from the sequential and simultaneous AD tasks and the plasticity metric is computed from the relationship between the two. The relationship between performances on these two tasks was first described in 2008 and the simultaneous AD task performance has been demonstrated to be approximately equal to task performance in the sequential AD task in healthy populations [34]. The important dimension is the relative performance of simultaneous AD task performance to sequential AD task performances: the worse the performance of the simultaneous task relative to the sequential task, the worse the plasticity metric. Task performance on simultaneous AD is greatly compromised in populations with hypersensitivity (e.g., migraine) [52]. Additionally, the simultaneous AD task demonstrates a correlation with data from non-human primates, and degradation in lateral inhibition has been demonstrated both in vivo and in vitro in cortical states that mimic hypersensitivity. In this case study, there was a substantial improvement in performance in this metric in the time course post-concussion [53-55].
Temporal order judgment
Temporal order judgment (TOJ) was much higher than normative values for the subject initially but returned to normative values over the time-frame studied. This metric is largely attributed to pathways in the frontal, pre-frontal and parietal cortex [56-61]. The connectivity metric is computed by determination of relative performance of the TOJ carrier (TOJ with an illusory conditioning stimulus that normally makes it more difficult to perform the task), and a poor score on connectivity reflects that the illusory confound had little or no effect on TOJ performance. In other words, absence of the effect of the illusion causes the concussed individual to outperform non-concussed individuals on the TOJ task (this was the case for a group study of concussed vs. non-concussed individuals) [26].
Time perception
The finding that the duration discrimination (DD) was slow on Ms K’s D-18 test was not unexpected. In a previous study reporting on assessment of concussion of student athletes, the ability to accurately determine which of two stimuli has a longer temporal duration, had a less of an impact on concussed individuals. Ms ‘K’s’ timing perception (or DD) showed notable improvement over the time course examined in this case study. The ability of an individual to differentiate which of two stimulus epochs is longer in duration has been attributed to cerebellar-cortical circuitry: in studies in which TMS was used to block activity in the cerebellum, timing perception task performance was greatly reduced or eliminated regardless of which sensory modality was used [62].
Connectivity
In the presence of a conditioning stimulus, healthy subjects do worse on the TOJ task because of the connectivity between adjacent cortical regions [30]. The conditioning stimulus is hypothesized to engage adjacent and near-adjacent cortical ensembles that are exhibit stimulus evoked activity [26,30,52]. First described in 2007 [30], the connectivity metric was used to demonstrate a lower than normal functional connectivity in individuals with autism [63]. This demonstration paralleled previous histological post-mortem findings that predicted lower than normal inhibition in autism [64]. In short, the decreased performance in the TOJ task in healthy controls with the illusory conditioning stimulus was not evident in the autism population, indicating lower than normal connectivity. Subsequently, similar findings were demonstrated in other populations that (predictably) had lower than normal connectivity (e.g., individuals with migraine [52]; concussed individuals [26]. In these populations, individuals impacted by some compromise of functional connectivity performed better on the TOJ task in the presence of the conditioning stimulus because the lack of synchronized activity led to decoupling of activity at the two-digit representations. This lower than normal connectivity can be seen in the results of Ms ‘K’ where her TOJ was nearly 80 ms greater than the normative range on D-18 but as her concussion recovered the TOJ returned towards the normative range (D-48). Additional evidence of the role of synchronized activity between adjacent cortical ensembles in task performance were obtained in studies with TMS (in which synchronized activity was decoupled [65], MEG studies that demonstrated a lack of synchronization in local cortical ensembles that paralleled task performance [66] and non-human primate studies [67-69].
Summary
In summary, using the somatosensory system provides access to a diagnostic system for overall cortical health by enabling access to a somatotopic organization for evoking cortical-cortical interactions in adjacent, or near adjacent, regions of the brain [26]. In addition, the somatosensory system can control for ambient environment noise (tactile stimuli vs auditory or visual stimuli), is highly integrated with the pain system and alterations in sensory input occur in parallel with alterations on systemic cortical alterations enabling ‘sampling’ from the center of the brain [26]. These neurosensory assessments had been previously described as sensitive to systemic cortical alterations and are successful in differentiating groups of concussed vs. non-concussed individuals at a high confidence level [26]. The overall finding of the case study was that Ms ‘K’ greatly improved in all metrics, indicating a network-wide improvement in performance and return to normative function over the time range studied post-concussion. The potential for the Brain Gauge to be utilized as a tool to individually assess the recovery of concussions needs to be researched further.
Conclusion
The identification and monitoring of the different facets of the brain gauge enabled the recovery of Ms ‘K’ to be recorded, and the provision of a visual display to show the progress of her recovery was beneficial to Ms ‘K’. The findings that the TOJ, timing perception, fatigue, plasticity and duration discrimination were outside normative levels was not unexpected and, with appropriate cognitive and physical rest these returned towards the normative range. The multiparametric approach of cortical metrics was sensitive to the degree of recovery and the diversity of symptoms that Ms ‘K’ sustained from her head injury. Further research is warranted to evaluate the Brain Gauge for the use of individual concussion recovery assessments.
Practical Implications
The Brain Gauge somatosensory system enables rapid visual identification of where the individual is in their recovery from a concussive injury and warrants further investigation as a tool to help monitor physiological recovery from concussion.
Acknowledgements
Thanks are given to Ms ‘K’ and her manager for agreeing to trial the new Brain Gauge technology during her recovery. Thanks are given to cortical metrics for the Brain Gauge system to trial in the clinical setting.
References
- Quintana L (2016) Second impact syndrome in sports. World Neurosurg 91: 647-649.
- McLendon L, Kralik S, Grayson P (2016) The controversial second impact syndrome: A review of the literature. Pediatr Neurol 62: 9-17.
- Broglio S, Puetz T (2008) The effect of sport concussion on neurocognitive function, self-report symptoms and postural control: A meta-analysis. Sports Med 38: 53-67.
- Dougan BK, Horswill MS, Geffen GM (2013) Athletes age, sex, and years of education moderate the acute neuropsychological impact of sports-related concussion: A meta-analysis. J Int Neuropsychol Soc 20: 64-80.
- Williams RM, Puetz TW, Giza CC, Broglio SP (2015) Concussion recovery time among high school and collegiate athletes: A systematic review and meta-analysis. Sports Med 45: 893-903.
- Kontos AP, Braithwaite R, Dakan S, Elbin RJ (2014) Computerized neurocognitive testing within one week of sport-related concussion: Meta-analytic review and analysis of moderating factors. J Int Neuropsychol Soc 20: 324-332.
- McCrea M, Guskiewicz KM, Marshall SW, Barr W, Randolph C, et al. (2003) Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA 290: 2556-2563.
- Nelson L, Janecek J, McCrea M (2013) Acute clinical recovery from sport-related concussion. Neuropsychol Rev 23: 285-299.
- McCrory P, Meeuwisse W, Dvorak J, Aubry M, Bailes J, et al. (2017) 5th International Conference on Concussion in Sport (Berlin). Br J Sports Med 51: 838-847.
- Henry LC1, Elbin RJ, Collins MW, Marchetti G, Kontos AP (2016) Examining recovery trajectories after sport-related concussion with a multimodal clinical assessment approach. Neurosurg 78: 232-241.
- Hang B, Babcock L, Hornung R, Ho M, Pomerantz WJ (2015) Can computerized neuropsychological testing in the emergency department predict recovery for young athletes with concussions. Pediatr Emerg Care 31: 688-693.
- Baker J, Leddy J, Darling S (2016) Gender differences in recovery from sports-related concussion in adolescents. Clin Pediatr 55: 771-775.
- Kamins J, Bigler E, Covassin T, Henry L, Kemp S (2017) What is the physiological time to recovery after concussion? A systematic review. Br J Sports Med 51: 935-940.
- Meier TB, Bellgowan PS, Singh R, Kuplicki R, Polanski DW, et al. (2015) Recovery of cerebral blood flow following sports-related concussion. JAMA Neurology 72: 530-538.
- Maugans T, Farley C, Altaye M (2012) Pediatric sports-related concussion produces cerebral blood flow alterations. Pediatrics 12: 928-937.
- Slobounov S, Sebastianelli W, Hallett M (2012) Residual brain dysfunction observed one year post-mild traumatic brain injury: Combined EEG and balance study. Clin Neurophysiol 123: 1755-1761.
- Slobounov S, Cao C, Sebastianelli W (2009) Differential effect of first versus second concussive episodes on wavelet information quality of EEG. Clin Neurophysiol 120: 862-867.
- Gosselin N, Theriault M, Leclerc S (2006) Neurophysiological anomalies in symptomatic and asymptomatic concussed athletes. Neurosurg 58: 1151-1161.
- Cao C, Slobounov S (2011) Application of a novel measure of EEG non-stationarity as ‘Shannon- entropy of the peak frequency shifting’ for detecting residual abnormalities in concussed individuals. Clin Neurophysiol 122: 1314-1321.
- Powers KC, Cinelli ME, Kalmar JM (2014) Cortical hypoexcitability persists beyond the symptomatic phase of a concussion. Brain Inj 28: 465-471.
- De Beaumont L, Mongeon D, Tremblay S (2011) Persistent motor system abnormalities in formerly concussed athletes. J Athl Train 46: 234-240.
- Merritt V, Meyer J, Arnett P (2015) A novel approach to classifying postconcussion symptoms: The application of a new framework to the Post-Concussion Symptom Scale. J Clin Exp Neuropsyc 37: 764-775.
- Guskiewicz K, Register-Mihalik J, McCrory P (2013) Evidence-based approach to revising the SCAT: introducing the SCAT3. Br J Sports Med 47: 289-293.
- Shehata N, Wiley JP, Richea S (2009) Sport concussion assessment tool: Baseline values for varsity collision sport athletes. Br J Sports Med 43: 730-734.
- Puts N, Edden R, Wodka E (2013) A vibrotactile behavioral battery for investigating somatosensory processing in children and adults. J Neurosci Methods 218: 39-47.
- Tommerdahl M, Dennis R, Francisco E (2016) Neurosensory assessments of concussion. Mil Med 181: 45-50.
- Tommerdahl M, Favorov O, Whitsel B (2010) Dynamic representations of the somatosensory cortex. Neurosci Biobehav Rev 34: 160-170.
- Tommerdahl M, Hester KD, Felix ER (2005) Human vibrotactile frequency discriminative capacity after adaptation to 25 Hz or 200 Hz stimulation. Brain Res 1057: 1-9.
- Tommerdahl M, Tannan V, Cascio CJ (2007) Vibrotactile adaptation fails to enhance spatial localization in adults with autism. Brain Res 17: 1154.
- Tommerdahl M, Tannan V, Zachek M (2007) Effects of stimulus-driven synchronization on sensory perception. Behav Brain Funct 3: 61.
- Zhang Z, Francisco E, Holden J (2011) Somatosensory information processing in the aging population. Front Aging Neurosci 3: 18.
- Tannan V, Simons S, Dennis RG (2007) Effects of adaptation on the capacity to differentiate simultaneously delivered dual-site vibrotactile stimuli. Brain Res 1186: 164-170.
- Francisco E, Tannan V, Zhang Z (2008) Vibrotactile amplitude discrimination capacity parallels magnitude changes in somatosensory cortex and follows Weber’s Law. Exp Brain Res 191: 49-56.
- Zhang Z, Tannan V, Holden J (2008) A quantitative method for determining spatial discriminative capacity. BioMed Eng Online 7: 1-8.
- Donders FC (2009) Over de snelheid van psychische processen. Onderzoekingen gedaan in het Physiologische Laboratorium der Utrechtsche Hoogeschool, On the speed of mental processes. Acta Psychologica 1868: 412-431.
- MacDonald SWS, Nyberg L, Bäckman L (2006) Intra-individual variability in behavior: links to brain structure, neurotransmission and neuronal activity. Trends Neurosci 29: 474-480.
- Tamm L, Narad ME, Antonini TN (2012) Reaction time variability in ADHD: A review. Neurotherapeutics 9: 500-508.
- Ruesch J (1944) Dark adaptation, negative after images, tachistoscopic examinations and reaction time in head injuries. J Neurosurg 1: 243-251.
- Van Zomeren AH, Deelman BG (1978) Long-term recovery of visual reaction time after closed head injury. J Neurol Neurosurg Psychiatry 41: 452-457.
- MacFlynn G, Montgomery EA, Fenton GW (1984) Measurement of reaction time following minor head injury. J Neurol Neurosurg Psychiatry 47: 1326-1331.
- Stuss D, Stethem L, Higenholtz H (1989) Reaction time after head injury: fatigue, divided and focused attention, and consistency of performance. J Neurol Neurosurg Psychiatry 52: 742-748.
- Ponsford J, Kinsella G (1992) Attentional deficits following closed-head injury. J Clin Exp Neuropsychol 14: 822-838.
- Hetherington CR, Stuss DT, Finlayson MAJ (1996) Reaction time and variability 5 and 10 years after traumatic brain injury. Brain Inj 10: 473-486.
- Zahn TP, Mirsky AF (1999) Reaction time indicators of attention deficits in closed head injury. J Clin Exp Neuropsychol 21: 352-367.
- Warden D, Bleiberg J, Cameron K (2001) Persistent prolongation of simple reaction time in sports concussion. Neurology 57: 524-526.
- Collins M, Iverson G, Lovell M (2003) On field predictors of neuropsychological and symptom deficit following sports-related concussion. Clin J Sports Med 13: 222-229.
- Sarno S, Erasmus LP, Lipp B (2003) integration after traumatic brain injury: A reaction time study between pairings of vision, touch and audition. Brain Inj 17: 413-426.
- Willison J, Tombaugh TN (2006) Detecting simulation of attention deficits using reaction time tests. Arch Clin Neuropsychol 21: 41-52.
- Niogi SN, Mukherjee P, Ghajar J (2008) Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: A 3T diffusion tensor imaging study of mild traumatic brain injury. Am J Neuroradiol 29: 967-973.
- Gould JA, Ciuffreda KJ, Yadav NK (2013) The effect of retinal defocus on simple eye-hand and eye-foot reaction time in traumatic brain injury (TBI). Brain Inj 27: 1643-1648.
- Eckner JT, Richardson JK, Kim H (2015) Reliability and criterion validity of a novel clinical test of simple and complex reaction time in athletes. Percept Mot Skills 120: 841-859.
- Nguyen R, Ford S, Calhoun A (2013) Neurosensory assessments of migraine. Brain Res 1498: 50-58.
- Juliano S, Whitsel B, Tommerdahl M (1989) Determinants of patchy metabolic labeling in the somatosensory cortex of cats: a possible role for intrinsic inhibitory circuitry. J Neurosci 9: 1-12.
- Juliano SL, Code RA, Tommerdahl M (1993) Development of metabolic activity patterns in the somatosensory cortex of cats. J Neurophysiol 70: 2117-2127.
- Kohn A, Metz C, Quibrera M (1999) Functional neocortical microcircuitry demonstrated with intrinsic signal optical imaging in vitro. Neuroscience 95: 51-62.
- Milner B, Corsi P, Leonard G (1991) Frontal-lobe contribution to recency judgements. Neuropsychologia 29: 601-618.
- Milner B, Petrides M, Smith M (1985) Frontal lobes and the temporal organization of memory. Hum Neurobiol 4: 137-142.
- Takahashi T, Kansaku K, Wada M (2013) Neural correlates of tactile temporal-order judgment in humans: An fMRI study. Cereb Cortex 23: 1952-1964.
- Wittmann M, Burtscher A, Fries W (2004) Effects of brain-lesion size and location on temporal-order judgment. Neuroreport 15: 2401-2405.
- Vakil E, Weise M, Enbar S (1997) Direct and indirect memory measures of temporal order: Younger versus older adults. Int J Aging Hum Dev 45: 195-206.
- Jurado M, Junque C, Vendrell P (1998) Overestimation and unreliability in "feeling-of-doing" judgements about temporal ordering performance: Impaired self-awareness following frontal lobe damage. J Clin Exp Neuropsychol 20: 353-364.
- Koch G, Oliveri M, Caltagirone C (2009) Neural networks engaged in milliseconds and seconds time processing: Evidence from transcranial magnetic stimulation and patients with cortical or subcortical dysfunction. Philos Trans R Soc Lond B Biol Sci 364: 1907-1918.
- Tommerdahl M, Tannan V, Holden J (2008) Absence of stimulus-driven synchronization effects on sensory perception in autism: Evidence for local underconnectivity? Behav Brain Funct 24: 19.
- Casanova MF, Buxhoeveden D, Gomez J (2003) Disruption in the inhibitory architecture of the cell minicolumn: Implications for autisim. Neuroscientist 9: 496-507.
- Lee K, Jacobs M, Asmussen M (2013) Continuous theta-burst stimulation modulates tactile synchronization. BMC Neurosci 14: 89.
- Khan S, Michmizos K, Tommerdahl M (2015) Somatosensory cortex functional connectivity abnormalities in autism show opposite trends, depending on direction and spatial scale. Brain 138: 1394-1409.
- Forshey TM (2013) Neural basis of the neurological diagnostic power of vibrotactile sensory testing North Carlonia: The University of North Carolina at Chapel Hill.
- guyen R, Forshey T, Holden J (2014) Vibrotactile discriminative capacity is impacted in a digit-specific manner with concurrent unattended hand stimulation. Exp Brain Res 232: 3601-3612.
- Tannan V, Whitsel BL, Tommerdahl M (2006) Vibrotactile adaptation enhances spatial localization. Brain Res 1102: 109-116.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences