Urokinase in the Management of Relapsing Peritoneal Dialysis-Related Peritonitis
Sajeda Youssouf*, Lilian Ekeh and Stanley L Fan
Department of Renal Medicine and Transplantation, Barts Health NHS Trust, London, UK
- *Corresponding Author:
- Sajeda Youssouf
Department of Renal Medicine and Transplantation, The Royal London Hospital, Whitechapel, London E1 1BB, UK.
Tel: +44 20 359 42674
E-mail: sajedayoussouf@gmail.com
Received date: January 03, 2017; Accepted date: January 29, 2017; Published date: January 31, 2017
Citation: Youssouf S, Ekeh L, Fan SL. Urokinase in the Management of Relapsing Peritoneal Dialysis-Related Peritonitis. Jour Ren Med. 2017, 1:1.
Abstract
Background: Peritonitis remains the commonest cause of morbidity in patients undergoing peritoneal dialysis. Relapse may be related to catheter colonisation with bacterial biofilm, and requires PD catheter exchange or removal. We changed our treatment protocols for relapsing peritonitis to include IP Urokinase in February 2014 and report a non-randomised single centre retrospective before and after study of clinical outcomes.
Methods and findings: We compared outcomes of 12 patients with relapse of peritonitis treated with Urokinase and antibiotics with 14 historical controls who received standard antibiotic treatment prior to the policy change. All patients with relapsing peritonitis between 1st January 2011 and 31st December 2015 were included.
Results: We identified 28 patients with relapsing peritonitis. Two patients died from causes unrelated to infection. 12 patients were treated with Urokinase and antibiotics and 14 received antibiotics alone (control group). Demographics were similar between the Urokinase and control group. 6/14 in the control group and 11/12 in the Urokinase group achieved complete cure (p=0.03). Refractory peritonitis occurred in 4/14 controls and 1/12 Urokinase group. 4/14 control patients relapsed.
Conclusions: The gold standard treatment for relapsing peritonitis is currently catheter exchange. This however is invasive and resource-intensive. This small study demonstrates significant improvement in outcomes of relapsing peritonitis by adding Urokinase lock to standard antibiotic therapy when compared to historical controls, without the need for catheter exchange.
Keywords
Peritonitis; Peritoneal dialysis; Urokinase; Antibiotic therapy
Introduction
Despite advances in Peritoneal Dialysis (PD) technology and patient training PD-related peritonitis remains the commonest cause of morbidity. A recent 3-year UK renal registry analysis found 4894 PD-related admissions to hospital, resulting in 53,671 bed days and 220 deaths (Fotheringham J; unpublished data, personal communication). The ISPD recommends a maximum peritonitis rate of 1 infection per 18 patient months. The UK average is currently 1 in 25 patient months, comparable with that in Australia and New Zealand (1 in 30) [1], but lagging behind other international centres, which have demonstrated that rates of 1 in 60 or even lower are achievable [2].
Although the majority of infections respond to antibiotic therapy, relapse (defined as another episode of peritonitis within 4 weeks of completion of previous therapy with the same organism or “sterile” episode [3] is not uncommon. Increasingly, relapsing peritonitis is recognised as a distinct clinical entity from repeat or recurrent peritonitis, with different causative organisms and a different pathophysiological process [4,5]. There is evidence that having an episode of peritonitis increases the risk of further infection [6]. Potential causes include an inadequate intraperitoneal immune response to infection and repeated poor exchange technique. Colonization of the PD catheter by biofilm is also often implicated as a cause for relapsing PD peritonitis [7]. Bacteria start to form biofilm within 48 hours of catheter placement [6]. This biofilm consists predominantly of gram positive cocci in a fibrillar matrix [6]. It has been suggested that bacteria penetrate the glycocalyx matrix and become difficult to clear with antibiotics alone. Intraperitoneal (IP) Urokinase may disrupt biofilms and permit more efficacious antibiotic action. Whilst simultaneous catheter removal and replacement was found to be superior to IP Urokinase in reducing relapsing peritonitis episodes [8], the role of Urokinase remains unclear for patients that decline to undergo PD catheter exchange.
The PD peritonitis protocol at Barts Health NHS Trust was to treat patients with relapsing peritonitis with 2 appropriate antibiotics (based on culture results) for 21 days. From the 1st of Feb 2014, intraperitoneal Urokinase was added to the treatment protocol for relapsing peritonitis.
We have performed a retrospective study using historical controls to examine the impact of our policy chang e.
Methods
We examined outcomes of patients who had a relapse of PD related peritonitis from 1st Jan 2011 to 31st Dec 2015.
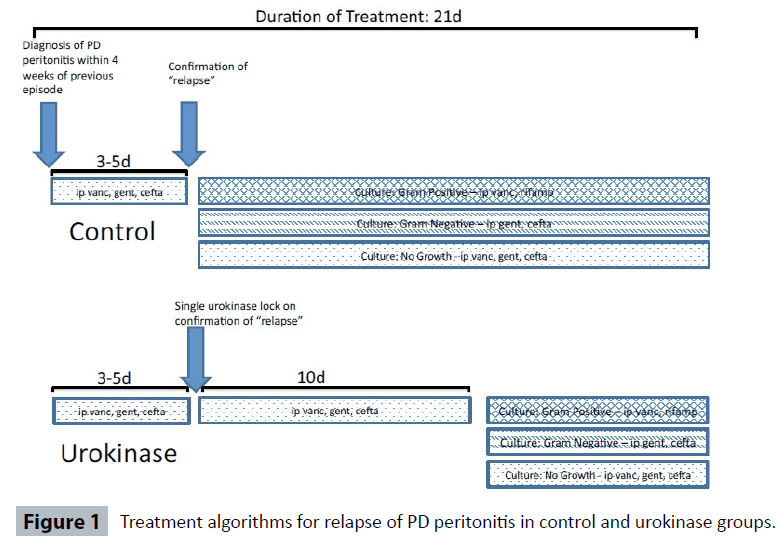
Diagnosis of peritonitis was made according to ISPD guidelines [3]. Empiric antibiotic treatment was IP vancomycin, gentamicin and ceftazidime. Treatment continued with IP vancomycin and oral rifampicin if gram-positive organisms were cultured (guided by sensitivity and resistance reporting). IP gentamicin and ceftazidime was used for gram-negative infections. Vancomycin, gentamicin and ceftazidime were continued if there was no growth. Dosing of antibiotics was in accordance to ISPD guidelines and has been described in a previous study [9].
Prior to 1 February 2014, if a relapse of peritonitis was confirmed, empiric antibiotics were continued for 3-5 days until culture and sensitivities were known, with treatment continued thereafter as dictated by culture results for a total of 21 days.
After 1 February 2014, patients with confirmed relapse of peritonitis were treated with a single Urokinase catheter “lock” of 5,000iu made up to 5mLs. After 2 hours, the PD catheter was aspirated and patients underwent a single rapid exchange and restarted empiric antibiotics for 10 days. After 10 days, antibiotics continued as dictated by culture results until a total of 21 days of treatment (Figure 1).
The outcome for an episode of relapse was assigned contemporaneously as:
1. Cured: infection cleared with treatment and no further peritonitis within 4 weeks.
2. Relapsed/recurrent: infection cleared with treatment but another peritonitis developed within 4 weeks irrespective of causative organism.
3. Refractory: If PD catheter was removed because of persistent cloudy PD effluent after 5 days of treatment.
Results
There were a total of 28 episodes of relapse PD peritonitis during the study period. Two patients died whilst receiving treatment for peritonitis (1 withdrew from dialysis, 1 died from a cardiac cause that was not felt to be directly related to infection). Both were control patients and, as their deaths were not felt to be related to infection, were excluded from analysis.
Of the remaining patients, 14 were control and 12 were treated with Urokinase. There were no significant differences in patient demographics (Table 1). There was a trend to longer PD vintage in the urokinase-treated patients, but this did not reach statistical significance.
| Control | Urokinase | p-value | |
|---|---|---|---|
| Number | 14 | 12 | NS |
| Age (yrs) | 61.3 (4.0) | 67.5 (3.8) | NS |
| Male Gender (%) | 80 | 67 | NS |
| Diabetic Mellitus (%) | 43 | 50 | NS |
| PD vintage (months) | 15.4 (3.9) | 27.1 (7.8) | NS |
| Previous Peritonitis episodes | 0.6 (0.2) | 0.8 (0.4) | NS |
| Previous Peritonitis rate | 0.05 (0.02) | 0.03 (0.01) | NS |
| Ethnicity (%) | |||
| White | 50 | 33 | NS* |
| Black | 29 | 25 | |
| Asian | 21 | 42 | |
| Organism causing Peritonitis (%) | |||
| Gram Positive | 36 | 25 | NS* |
| Enterococcus | 7 | 17 | |
| Gram Negative (excl. Enterococci) | 0 | 17 | |
| No Growth | 57 | 42 | |
| PD effluent WCC at Day 1 of relapse | 1840 (1100) | 1789 (684) | NS |
| Outcome of Treatment | |||
| Cleared | 6 | 11 | 0.03* |
| Relapsed within 4 weeks | 4 | 0 | |
| Refractory to treatment | 4 | 1 | |
| Values are Mean (SEM), WCC=White Cell count, p-value by Student t-test except * by Chi Square. | |||
Table 1 Details of patients treated with relapsing peritonitis.
There were no cases of staphylococcus aureus peritonitis and only 1 case of relapsed pseudomonas peritonitis (urokinase group) during the study period. This was undoubtedly because our policy is to remove PD catheters if peritonitis is caused by these organisms. There were no statistically significant differences in types of organisms grown (Table 1).
Cure occurred in 6/14 control and 11/12 Urokinase patients. There were 4 relapses in the control group. Refractory peritonitis occurred in 4/14 control and 1/12 Urokinase patients. The difference in outcomes was statistically significant (p=0.03 by Chi Square test).
Discussion
It has been demonstrated in laboratory studies that peritoneal dialysis induces a state of hypercoagulation and relative hypo fibrinolysis in the peritoneum, resulting in accelerated turnover of fibrin. This state is worse in peritonitis, with an imbalance between coagulation and fibrinolysis. However, there is evidence that there is reduced TPA production by peritoneal mesothelial cells in the presence of inflammatory mediators, resulting in an imbalance between coagulation and fibrinolysis in acute peritonitis. Urokinase activates cleavage of plasminogen to plasmin, promoting degradation of fibrin. It is this principle, and the potential benefit of Urokinase in enabling antibiotics to better penetrate biofilm on catheters, that underpins the use of Urokinase in this context.
However, the evidence that Urokinase improves outcomes of relapsing peritonitis is poor. Case reports and single centre uncontrolled series [10] showed promising results but results in controlled trials have been more mixed. Randomised controlled trials conducted to study Urokinase were not restricted to relapsing peritonitis [11], or included patients with refractory peritonitis [12,13]. Only one was specific to patients with a relapse of peritonitis [8]. This showed that PD catheter exchange resulted in a lower re-infection rate compared to urokinase/antibiotics. Current guidelines recommend catheter exchange for relapsing peritonitis, based on this study. Of note however, direct comparison is also complicated by differences in treatment protocols, with antibiotic treatment with a single antibiotic (once culture results were known) for a total of 10 days, rather than our standard practice of two antibiotics for 21 days. In clinical practice however, patients are often reluctant to undergo surgery that is performed under general anaesthetic with its associated risks. Hence we have accumulated a cohort of patients where we attempted medical treatment of their relapse peritonitis.
Conclusion
Despite the small study size, we found significantly improved ISPD-defined outcomes in patients treated with Urokinase lock. We acknowledge the limitations of our study. In addition to Urokinase, we introduced broad spectrum antibiotics for 10 days after Urokinase lock. The intention was to minimise risk of re-infection by organisms released from the biofilm, but it is possible this antibiotic escalation was the reason for the improved cure rate. There is also a risk with using historical controls, in that clinical practice may have evolved (e.g. we may have previously had a higher threshold for removing PD catheters on day 5). However, subsequent relapse is a hard end-point that is not open to interpretation and this was significantly lower. We also acknowledge that although not statistically different, control patients had higher infection rates that might signify greater biofilm colonisation burden. Nevertheless, we believe the results of our small retrospective study to be of interest and should spur further trials in this area.
Acknowledgements
SY and SF contributed to writing and revision of the manuscript. LE collected the data. SF designed the study and analysed the data.
No additional grants, support or funding was received for the production of this manuscript.
Competing and Conflicting Interests
SF has received an honorarium and travel expenses to give lectures on behalf of Baxter Healthcare.
References
- ANZDATA. ANZDATA Registry (2015) 37th Report, Chapter 5: Peritoneal Dialysis. Australia and New Zealand Dialysis and Transplant Registry, Adelaide, Australia. Available at: https://www.anzdata.org.au.
- Piraino B, Bernardini J, Brown E, Figueiredo A, Johnson DW, et al. (2011) ISPD position statement on reducing the risks of peritoneal dialysis-related infections. Perit Dial Int. 31: 614-630.
- Li PK, Szeto CC, Piraino B, De Arteaga J, Fan S, et al. (2016) ISPD Peritonitis Recommendations: 2016 Update on Prevention and Treatment. Perit Dial Int 36: 481-508.
- Burke M, Hawley CM, Badve SV, McDonald SP, Brown FG, et al. (2011) Relapsing and recurrent peritoneal dialysis-associated peritonitis: A multicenter registry study. Am J Kidney Dis. 58: 429-36.
- Szeto CC, Kwan BC, Chow KM, Law MC, Pang WF, et al. (2009) Recurrent and relapsing peritonitis: causative organisms and response to treatment. Am J Kidney Dis. 54: 702-710.
- Finkelstein ES, Jekel J, Troidle L, Gorban-Brennan N, Finkelstein FO, et al. (2002) Patterns of infection in patients maintained on long-term peritoneal dialysis therapy with multiple episodes of peritonitis. Am J Kidney Dis 39: 1278-1286.
- Read RR, Eberwein P, Dasgupta MK, Grant SK, Lam K, et al. (1989) Peritonitis in peritoneal dialysis: bacterial colonization by biofilm spread along the catheter surface. Kidney Int. 35: 614-621.
- Williams AJ, Boletis I, Johnson BF, Raftery AT, Cohen GL, et al. (1989) Tenckhoff catheter replacement or intraperitoneal Urokinase: a randomized trial in the management of recurrent continuous ambulatory peritoneal dialysis (CAPD) peritonitis. Perit Dial Int. 9: 65-67.
- Blunden M, Zeitlin D, Ashman N, Fan SL (2007) Single UK Center experience on the treatment of PD peritonitis--antibiotic levels and outcomes. Nephrol Dial Transplant. 22: 1714-1719.
- Pickering SJ, Fleming SJ, Bowley JA, Sissons P, Oppenheim BA, et al. (1989) Urokinase: a treatment for relapsing peritonitis due to coagulase-negative staphylococci. Nephrol Dial Transplant. 4: 62-65.
- Gadallah MF, Tamayo A, Sandborn M, Ramdeen G, Moles K (2000) Role of intraperitoneal urokinase in acute peritonitis and prevention of catheter loss in peritoneal dialysis patients. Adv Perit Dial 16: 233-236.
- Tong MK, Leung KT, Siu YP, Lee KF, Lee HK, et al. (2005) Use of intraperitoneal urokinase for resistant bacterial peritonitis in continuous ambulatory peritoneal dialysis. J Nephrol 18: 204-208.
- Innes A, Burden RP, Finch RG, Morgan AG (1994) Treatment of resistant peritonitis in continuous ambulatory peritoneal dialysis with intraperitoneal urokinase: A double-blind clinical trial. Nephrol Dial Transplant 9: 797-799.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences