Unmasking Plasma Membrane Blebbing
Godwin A Ponuwei*
Department of Biomedical Science, School of Life Sciences, University of Reading, Whiteknights Campus, Berkshire, UK
- *Corresponding Author:
- Godwin A Ponuwei
Department of Biomedical Science
School of Life Sciences, University of Reading
Whiteknights Campus, Berkshire, UK
Tel: +44 118 987 5123
E-mail: goddyponuwei@yahoo.com
Received date: October 21, 2017; Accepted date: October 30, 2017; Published date: November 10, 2017
Citation: Ponuwei GA. Unmasking Plasma Membrane Blebbing. J Biomed Sci Appl Vol. 1 No. 2:6.
Copyright: © 2017 Ponuwei GA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The eukaryotic cell plasma membrane is tightly connected to the underlying cytoskeletal network. When this close association is disrupted, the overlying plasma membrane detaches from the cortical actin and bulges out due to intracellular hydrostatic pressure-driven cytosolic fluid and lipids flow resulting in the formation of spherical, blister-like protrusions called blebs. Membrane blebs have been observed during normal biological and pathophysiological conditions including apoptosis, cytokinesis, and embryogenesis and cancer metastasis. Cell migration via membrane blebbing is fundamentally different from the lamellipodial-based mesenchymal migration that is characterized by actin filament polymerization. The aim of this review is to provide a general overview of the concept of plasma membrane blebbing. How cells utilize membrane blebs for motility as well as recent findings on the molecular regulators of bleb formation is discussed. A description of different processes and conditions in which membrane blebbing is utilized is also highlighted.
Keywords
Actin cortex; Blebs; Contractility; Cytoskeleton; Membrane; Protrusions
Abbreviations
DAPK: Death-associated protein kinase; DNA: Deoxyribonucleic acid; Dnd: Dead end protein; ECM: Extracellular matrix; FHOD1: Formin homology 2 domain containing 1; FMNL1: Formin-like protein 1; ERM: Ezrin, radixin, moesin; GAP: GTPase-activating protein; GEF: Guanine nucleotide exchange factor; LPA: Lysophosphatidic acid; MAT: Mesenchymal amoeboid transition; PIP2: phosphatidylinositol 4,5-bisphosphate; PGCs: Primordial germ cells; ROCK: Rho-associated coiledcoil kinase; SDF: Stromal cell-derived factor.
Introduction
The eukaryotic cell plasma membrane and the underlying actin cytoskeleton are tightly connected by linker proteins, particularly the ERM (ezrin, radixin, and moesin) family proteins, and this tight connectivity is critical for overall cellular integrity and signal transduction between the intracellular and extracellular compartments of the cell [1,2]. When the plasma membrane-cortex association is perturbed, the membrane gets detached from the actin cortex at the point of perturbation leading to the formation of hemispherical, blister-like herniation’s called blebs [3]. Plasma membrane blebs are free of cellular organelles [4], and their formation is attributed to either the depletion of the membrane-cortex linker proteins which results in weakening of adhesion between the membrane and the underlying actin cytoskeleton or a local breakage of cortex due to actomyosin contraction [5,6]. Membrane blebs usually appear and disappear asynchronously and haphazardly in a cyclical fashion within a very short interval of time [7]. Cell blebbing is a crucial event observed in both in vivo and in vitro settings in several biological processes including cytokinesis, cell spreading, apoptosis, embryogenesis and cell migration in mammals and other lower organisms [6,8-10]. Although, membrane blebs in migrating cells are analogous to, and serve similar purpose as other plasma membrane structures such as lamellipodia, filopodia, podosomes and invadopodia which are formed by polymerization of actin filaments [11-14], their formation is independent of actin polymerization. Similarly, cell migration via plasma membrane blebbing is not dependent on proteolytic degradation of the matrix in that the cells only squeeze through pre-existing orifices in three-dimensional matrices [15,16].
Cellular plasma membrane blebbing is an old phenomenon that dates back to the late 1910s when it was first observed in fibroblasts treated with solutions of different osmotic conditions [17]. It was later observed in cultures of newt tissues during mitosis [18]. Although observed for a very long time in different cell types [19,20], blebbing migration was not given much attention as focus was mainly on the lamellipodialbased migration which was considered the primary mode of cell migration. Over the past decades, blebbing has been associated with, and viewed only to be a hallmark of apoptosis during which dynamic blebs form at the execution phase of programmed-cell death [21]. Interestingly, bleb formation is also observed during necrosis during which cells are exposed to noxious environmental insults such as acute oxygen levels, deficient ATP (energy) levels and reactive oxygen species (free radicals) [22,23]. It is imperative to note that whereas bleb formation in both normal healthy cells and apoptotic cells is triggered by actomyosin contractility, necrotic blebs are not only bigger in size, their formation is independent of actomyosin contractility, but by ion influx. Similarly, whereas blebs in healthy and apoptotic cells dynamically expand and retract, necrotic blebs never retract their membranes [23].
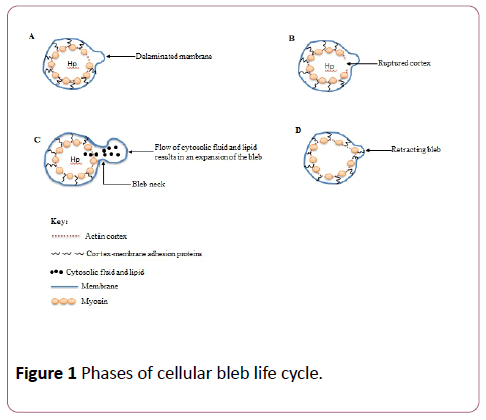
Life cycle of cellular bleb
The life cycle of a cellular bleb involves the following phases: bleb nucleation (initiation), expansion, stabilization and retraction [24] (Figure 1).
Bleb nucleation
Bleb formation is initiated by two mechanisms both of which could be triggered by extracellular stimuli such as experimental techniques involving two-photon laser ablation of cortical actin resulting in actomyosin-powered contractility of the actin cortex thereby creating a weaken cortexmembrane interaction [25]. This eventually leads to the detachment of the plasma membrane from the underlying cortex [10]. In the second mechanism, following actomyosin contractility, the actin cortex itself collapses, permitting cytosolic fluid and lipid to flow through the ruptured cortex and bulges the plasma membrane outwards giving rise to blebs [26]. A bleb forms during nucleation if a portion of the detached plasma membrane grows to a threshold size that is critical for blebbing, and this critical size is determined by cytoplasmic pressure, membrane tension and adhesion energy between the membrane and cortex [7].
Bleb expansion (Growth)
Bleb expansion, which is also refer to as the growth phase of the bleb cycle, persists for approximately between 5-30 seconds, and is caused by the generation of hydrostatic pressure in the cytoplasm due to actomyosin contractility thereby pulling out the plasma membrane from the actin cortex [2,22]. Apart from membrane delamination, bleb growth and expansion can also occur by the opening of creases on the plasma membrane from their original folded conformation via mechanisms not yet fully understood. [22]. The bleb becomes enlarged in volume as cytosolic fluid and lipid flow through the bleb neck into the bleb resulting in an increase in the surface area of the bleb [24]. The growth of blebs, unlike other cellular protrusions such as lamellipodia and filopodia is mediated by cytoplasmic pressure instead of polymerization of actin filaments, and an expanding bleb lacks actin cortex [27].
The mechanism(s) of cellular bleb growth is still under intense investigation. However, different models have been recently postulated to explain the how blebs grow and expand. One of such models is the coarse-grained model which proposed that the intracellular hydrostatic pressure generated in a porous elastic cytoplasm is produced by the action of a contractile gel-like actomyosin cortex, and that bleb expansion requires a threshold tensional force since the plasma membrane is resistant to distortion. Expansion of plasma membrane bleb would be impossible if the tensional force that exists at the cortex is below the threshold, irrespective of cortex rupture or weakened membrane-cortex adhesion [3,6]. On the other hand, when the threshold tensional force is exceeded, there is a corresponding increase in the size of blebs with rising tensional force [6]. A second model proposed for cellular bleb expansion is a mathematical computer-based approach that uses simulations to delineate the molecular mechanisms that regulate bleb formation and expansion [28,29]. This model adopted a holistic approach that investigated the individual contributions of the cytoplasm, cortex, cell membrane and cortex-membrane interaction (adhesion energy) to bleb formation and expansion. Unlike the coarse-grained model that gives only petite details about the molecular regulators of cellular blebbing, the computational model not only has the advantage of unraveling the cellular mechanics, but also gives a better insight into cellular bleb expansion at the molecular level [28,30]. A particle-based model that was previously proposed by using computer simulations showed that when the surface area of the plasma membrane is greater than that of the cortex, then cellular bleb formation and expansion is enhanced [29]. Similarly, by considering parameters such as matrix geometry and ECM adhesiveness, an agent-based model was recently utilized to investigate the dynamics of cell blebbing, and it was reported that the cytoplasmic pressure which drives the expansion of a cellular bleb is evenly distributed spatially [31].
Bleb stabilization
Bleb expansion gradually subsides as the rate of actomyosin contractility-dependent fluid and lipid influx becomes inadequate to sustain a continuous growth [32]. Thus, an expanding bleb gets to a stable point (bleb stabilization) before retracting their membranes [33].
Bleb retraction
Bleb retraction is preceded by reassembly of actin cortex beneath the bleb membrane. Although, the mechanisms responsible for the actin assemblage beneath the bleb membrane which subsequently results in bleb retraction are not clearly understood, it could be that there is occurrence of de novo polymerization of actin filaments by yet undiscovered actin nucleators, or that during the expansion phase, the bleb membrane might harbour very minute microscopically unnoticeable pieces of actin from the previous cortex, thus, giving rise to actin cortex repolymerization, an event that heralds the commencement of bleb retraction [34]. Plasma membrane bleb retraction is accompanied by successive events of protein recruitment. The first of such proteins to be recruited to the bleb membrane is ezrin, a member of the ERM (ezrin, radixin and moesin) membrane-cortex linker proteins [1,32]. Next, in a manner independent of ezrin recruitment and reassembly, there is the recruitment to bleb membrane of actin filaments, and this is finally followed by the sequential recruitment of tropomyosin, α-actinin and motor proteins, especially myosin II [32,34]. The bleb is then retracted by the newly formed cortex towards the cell body [10]. However, the fate of the bleb cortex upon full retraction to the cell body is not yet clearly understood. Retraction is the slowest phase of the bleb life cycle, and could last up to two minutes [7].
Plasma membrane blebbing is of great significance as it provides cell biologists the opportunity to investigate key mechanical parameters of the cell. Firstly, the detachment of the plasma membrane from the underlying cortical actin is being exploited to determine the strength of bonding between membrane and the cortex; on the other hand, cortex rupture itself can be exploited to investigate the tensional force created along the cortex, and finally, the fluxes of fluids and lipids during cellular blebbing has made it possible to study the flow of these cytosolic contents during blebbing [22].
Molecular regulators of plasma membrane bleb formation
The mechanisms used by cells to detect the sites of membrane bleb formation are not fully understood. However, it could involve the activity of mechanosensitive transmembrane ion channels which are able detect a breach in the integrity of the cortex-membrane interaction and this results in the generation of intracellular signalling cascades [34]. Different molecular regulators of plasma membrane and cytoskeletal dynamics have been delineated. It is known that a fragile interaction between the plasma membrane and actin cortex due to deficiency in actin polymerizing proteins such as filamin [24], talin [35] and depletion of membrane-cortex linker proteins such as the ERM (ezrin, radixin, moesin) proteins are factors largely responsible for the formation of plasma membrane blebs in different cell types [16,25] [36-38].
The 50 nm-2 μm thick actin cortex is a critical cytoskeletal component that lies beneath the plasma membrane and is mainly composed of actin filaments, contractile myosin II and actin-binding proteins. These actin-binding proteins play very crucial roles in mesenchymal-based cell migration through their involvement in the formation plasma membrane protrusions such as lamellipodia, filopodia, lobopodia and invadosomes (invadopodia and podosomes) which are utilized by cells in migration and invasion both in physiological processes and in pathological conditions [14,39]. In some cases, these actin polymerization-driven membrane protrusions could either act alone or synergistically to drive cell migration [40]. There is an inverse relationship between actin polymerization and plasma membrane bleb formation in that accumulation of actin polymerizing proteins is antagonistic to bleb formation as these proteins strengthen the interaction between the plasma membrane and the cortex. For instance, A375 melanoma cells deficient of the actin polymerizing protein, filamin, were reported to exhibit constitutive plasma membrane blebbing [32,41], suggestive of the converse correlation between actin filament polymerization and plasma membrane blebbing.
Formation of plasma membrane blebs, whether during apoptosis or in other biological processes is believed to be initiated by actomyosin contractility, and this contractile machinery itself is regulated by Rho- and ROCK-mediated phosphorylation of myosin light chain which phosphorylates myosin II [42,43]. This results in an upsurge in global and local hydrostatic pressure and subsequently, inflation of newly formed blebs [6,44]. The Rho family of small GTPases including Rho, Rac and Cdc42 are chief regulators of membrane ruffles and protrusions in different cells [39], [45-48]. These proteins usually switch between an active GTP-bound state mediated by guanine nucleotide exchange factors (GEFs) and an inactive GDP-bound state which is mediated by GTPase-activating proteins (GAPs) [49,50]. In response to platelets-derived growth factor (PDGF) stimulation, Rac1 can promote the formation of lamellipodia in fibroblasts [51] through direct interaction with SRA1 and WAVE1, two crucial constituents of the SCAR/Wave Regulatory Complex (WRC), and the resultant effect is the opening up in WAVE1 of a VCA (verprolin, cofilin and acidic homology) motif which causes Arp2/3 complex to be activated, leading to polymerization of actin monomeric units [52]. Through different signalling pathways, Cdc42 is involved in both mesenchymal, elongated, lamellipodial-based cell migration and plasma membrane bleb formation. In melanoma cells, DOCK10 act as a GEF, activating Cdc42 which signals through N-WASP and Pak2 to mediate rounded amoeboid movement reminiscent of blebbing migration [53]. Although different reports have implicated RhoA in mesenchymal elongated migration [50], it is now believed that the GTPase together with the other small Rho GTPases (RhoB and RhoC) can all activate downstream effector ROCK which is a main trigger of actomyosin contractility resulting in plasma membrane blebbing [47], [54-57]. ROCK triggers actomyosin contractility either by directly activating myosin light chain via phosphorylation or by inactivation via phosphorylation of the regulatory subunit of myosin phosphatase, MYPT1 [48,58].
RhoA and its downstream effector ROCK have been shown to be stimulated by nocodazole-induced depolymerization of microtubules which resulted in membrane blebbing in T cells [59]. Interaction of p27(kip1), the inhibitor of the cyclin/cyclindependent kinase complexes with RhoA resulted in the inhibition of the latter. Consistent with this, downregulation of p27(kip1) levels resulted in mesenchymal-to-amoeboid transition (MAT) with a corresponding plasma membrane blebbing [60]. The Rho GTPase-activating protein (GAP), FilGAP, specifically acts as a GAP towards Rac, antagonising lamellipodia formation and cell spreading mediated by Rac [61]. Consistent with this role, overexpression of FilGAP induced plasma membrane blebbing in a ROCK-dependent manner, while downregulation of endogenous FilGAP activity resulted in Rac-mediated mesenchymal morphology in carcinoma cells [62,63].
The Death-associated protein kinase (DAPK) is a serine/ threonine cytoskeletal kinase whose activity is regulated by calcium and calmodulin [64]. This kinase plays opposing roles in plasma membrane blebbing, and might probably function in a cell type-specific manner. Upon overexpression, DAPK stimulated the phosphorylation of myosin light chain to induce membrane blebbing in different cell types [34], [65-67]. On the contrary, in response to hydrogen peroxide activation of the ERK pathway, DAPK phosphorylates tropomyosin-1 to positively regulate the formation and stabilization of stress fibres thereby inhibiting plasma membrane blebbing [68].
The cell-cell adhesion molecule, E-cadherin, stimulates Rac1 to induce polymerization of actin filaments resulting in forward migration via lamellipodial membrane protrusions [69]. Interestingly, E-cadherin mediated cell-cell adhesion was reported to be required for plasma membrane blebbing induced by αE-catenin and ezrin depletion in zebrafish. According to this proposition, cells in the external layer become increasingly adhesive as a result of increased Ecadherin levels, and this subsequently resulted in weakening of the membrane-cortex interaction [37].
The involvement of lipid signaling molecules in plasma membrane blebbing has been extensively studied in different cell lines [33], [70,71]. It was recently reported that the lipid signalling phospholipase D2 (PLD2) but not PLD1 signals through the versatile intracellular second messenger, phosphatidic acid in a mechanism that involves the lysophosphatidic acid receptor (LPAR)-ROCK signalling pathway to mediate plasma membrane blebbing in human fibrosarcoma HT1080 cancer cell line [33]. In a similar vein, the LPAR-ROCK axis was implicated in ATP-induced blebbing of osteoclasts [72]. In Dictyostelium cell, plasma membrane blebbing was observed following delocalization of phosphatidylinositol 3,4,5-triphosphate (PIP3) from the membrane in response to microtubule depolymerisation [71]. Depletion of PIP2 levels in human umbilical vein endothelial cells (HUVEC) significantly enhanced fixation-induced blebbing, suggestive of the role of PIP2 in stabilizing the membranecortex interaction [70]. PIP2 was reported to stimulate the activity of the ERM proteins, and consistent with this, loss of PIP2 activity correlated with detachment of the membrane from the actin cortex [73,74]. How PIP2 exert its effect on cytoskeletal dynamics is not clearly understood, however, the second messenger may function in a cell type-specific manner in that inhibition of PIP2 activity was unable to prevent actin filaments repolymerization during retraction of blebs in filamin-deleted cells [32].
There are ample reports on the involvement of formins in plasma membrane blebbeing. Transfection of cells to overexpress the scaffolding protein, diaphanous-interacting protein (DIP), showed that the protein interacts with and negatively regulates the activity of the formin mDia2, leading to plasma membrane blebbing [75]. This demonstrates the role of mDia2 as a membrane-cortex linker protein that prevents the dissociation of the plasma membrane from the cortex, thus, stabilizing the actin cortex. A similar membranecortex stabilizing role was attributed to the overexpression of another formin, FHOD1 (FH2 domain-containing protein 1) in that only a decrease in the size of plasma membrane blebs resulting from ROCK signaling was observed, whereas bleb numbers was elevated [76]. However, contrary to the aforementioned bleb-inhibiting roles of the formins, in a manner dependent on its N-terminal myristoylation but not on ROCK signalling, the formin-like protein 1 (FMNL1) induced plasma membrane blebbing [77], nonetheless, the molecular mechanisms of FMNL1-induced membrane blebbing need to be fully elucidated. Overexpression of constitutively active Met, fluorescent Met as well as stimulation of Met/hepatocyte growth factor signalling pathway resulted in plasma membrane blebbing in various cell lines including MDA-MB-231 breast cancer and HEK293 cells [78], invasive Moloney-sarcoma-virustransformed Madin-Darby canine kidney (MDCK MSV-MDCKINV) cells [79] and small cell lung cancer cells [80]. Stimulation with substance P, a neurokinin 1 receptor (NK1R) agonist, of HEK293 cells and U373MG astrocytoma cells overexpressing the receptor resulted in dynamic non-apoptotic blebbing [81,82].
Another protein that was reported to be critical for induction of membrane blebbing is Dead end (Dnd) [25]. This protein binds RNA and promotes mRNAs translation by downregulating microRNA activity [83]. Knockdown of this protein in primordial germ cells of zebrafish resulted not only in reduction of actomyosin contractility-mediated plasma membrane blebbing, but also strengthens membrane-cortex interaction by up regulating the levels of the membrane-cortex linker protein, Annexin A5b [25].
Bleb polarization and migration
It has been established that plasma membrane blebs are initiated by dissociation of the plasma membrane from the actin cortex. However, insights on how the blebs get polarized to the leading edge and subsequently translocate from one point to another are still very rudimentary. An increase in contractility of the membrane-cortex interaction with its resultant effect of weakening the integrity of this interaction at the leading edge of the cell, due to, for example, stimulation of cells with a chemoattractants such as cyclic AMP and folate [84] might be a reason for polarization and bleb-based migration, as observed in zebrafish germ cells [24,85]. A possible explanation for the waning membrane-cortex interaction could be that the ERM proteins which link the membrane and cortex [1] may be more concentrated at the cell rear thereby giving rise to dissociation of the membrane from the underlying cortex at the leading edge as evidenced in migrating Walker carcinoma cells [86,87].
Similarly, the leading edge may have more concentration of myosin molecules which generate more contractile force at this region than the cell rear, and this may result in local rupture of the actin cortex [26,88]. Increased intracellular calcium has been shown to precede blebbing in zebrafish germ cells and this generated the contractile force needed for cortex-membrane detachment, and subsequently, blebbing migration [85]. In addition to having more myosin concentration, the leading edge of blebbing cells is more pliable and younger than the cell rear, so blebbing mainly occurs at the front rather than the rear of the cell [22]. However, in some cases, during blebbing migration, myosin motors can be found both at the cell front [86] and rear [89]. Similarly, in some tumor cells, ROCK1, which mediate actomyosin contractility localizes to the trailing edge of migrating cells [90].
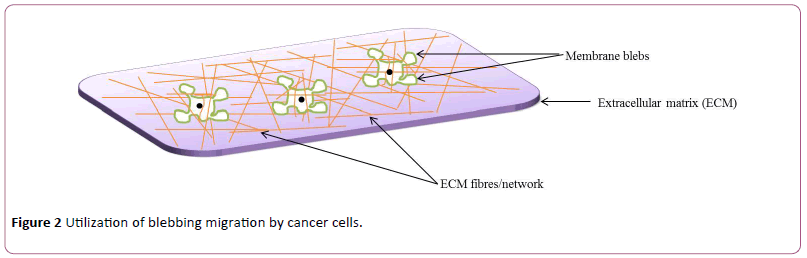
Usually, cell motility requires application of force by the cell body on the underlying substratum and this cause a change in the position and direction of the cell. In lamellipodial-based migration, the trailing edge first undergoes contraction; this is then linked together with interaction occurring between the underlying ECM and lamellipodia. In contrast, how cells migrate from one point to another via blebbing is not clearly understood. It is speculated that there might be some forms of weak interactions between focal adhesions and the ECM on one hand, and with neighboring cells on the other hand, thereby propelling the cell to migrate by blebbing [22,91]. Such focal adhesions having connected to the ECM and the forming actin cortex, permit not only contraction of the cell rear but also cause the detachment of the cell-ECM interaction, and this subsequently leads to translocation of the cell mass [92]. Similarly, a cell may translate blebbing into migration through a process called chimneying which is independent of matrix adhesion and occurs either in 2D or 3D environment where a cell becomes flattened, and migration is achieved by applying a force perpendicular to the ECM [24,93]. Unlike other membrane protrusions driven by actin polymerization, blebs can grow and expand from different directions of a cell [24] and in some cases of migrating cells, bleb retraction is not observed, rather, secondary blebs grow from existing ones [42,94]. Because blebbing is independent of matrix proteolysis, lesser energy is required for blebbing motility than lamellipodial-based mesenchymal migration [95]. Cells migrating via blebbing exhibit more plasticity which makes them to conform more quickly to the silhouette of their microenvironment, as the blebs are devoid of cortical actin as well as possess reduced membrane-cortex linker proteins at the leading edge [24] (Figure 2).
Blebbing in biological processes
Blebbing and apoptosis: Apoptosis is a form of programmed cell death which is crucial for growth and development of tissues and organs [96]. It is a normal homeostatic mechanism healthy cells use to get rid of defective and unwanted cells [97], and to prevent abnormal and excessive cell proliferation [98]. The execution phase of apoptosis is usually mediated by caspases and is associated with shrinkage of cell, DNA contraction, nuclear disintegration, and dynamic plasma membrane blebbing [97]. Although, membrane bleb formation is a commonly observed feature in apoptotic cells, and has been regarded as a hallmark of programmed cell death, not all cells undergo membrane blebbing during apoptosis, suggestive that it is not a general phenomenon [99,100]. It is not clear what roles blebbing plays in an apoptotic cell. However, it has been shown that membrane blebs are involved in trapping damaged cytosolic and membrane contents thereby providing a mechanism of resistance against injury-induced cell lysis and death [101]. Apoptosis-triggered exposure of bleb membrane proteins act as chemoattractants that help in recruitment of immune cells to site of damage [102]. Blebs aid in nuclear fragmentation during apoptosis because the presence of compressed chromatin and other cellular organelles lodged in the blebs help facilitates disintegration [22,99].
Blebbing and cell spreading: Spreading cells display dynamic plasma membrane blebs [103,104]. Cell spreading, just like cellular blebbing, is observed in biological processes such as cell migration, cell division and embryogenesis [9]. It involves interaction of cell with underlying substratum, and this interaction is positively regulated by membrane blebs in that blebs increase the surface area of adhesion of cells to their substrates thereby enhancing spreading [95]. However, in contrast, in endothelial cells subjected to chemical and osmotic perturbation, decreased blebbing activity correlated with increased cell spreading, an effect attributed first, to multiple attachment and retraction dynamics of bleb and then to the delay in lamellae formation in blebbing cells [9].
Blebbing and cell migration: Cell migration through threedimensional matrices was initially thought to be mediated primarily through the famed mesenchymal migration mode which is characterized by lamellipodia and filopodia formation. There is now ample evidence that different cells including Dictyostelium [105,106], immune cells [107,108] and tumour cells [33,47,109] migrate on flat surfaces or travel through 3D structures [10] by using membrane blebbing. It is also known that Walker carcinoma cell lines can migrate not only on 3D structures but also on 2D using blebbing [5,92]. In the context of cancer, the plasticity of metastatic cancer cells poses a great challenge in the treatment of the disease condition in that the cells are unaffected by anti-tumour agents, as they can switch between lamellipodial- and bleb-based motility [47,110]. In some cases, a cell migrating via blebbing does not totally exclude the possibility of utilizing the mesenchymal mode of migration that is associated with lamellipodia formation. For instance, cooperativity between actin-driven pseudopods and membrane blebs during chemotaxis of Dictyostelium has been reported [84]. Similarly, both blebs and lamellipodia are utilized during gastrulation of prechordal plate precursor cells of zebrafish in an alternating manner, and these membrane protrusions were found at different points of the leading edge on the cell membrane [36]. However, the more a cell bleb, the lesser the lamellipodia, and vice versa, and this is attributed to the differences in the mechanisms of the formation of these two membrane protrusion types [111].
Blebbing and cytokinesis: Blebbing is observed during cell division [112,113], particularly around the cleavage furrow probably due to disruption of interaction between plasma membrane and cortical actin at polar regions [22]. The exact role of blebs in cytokinesis remains obscure, however, it was speculated they might contribute to formation of actin cortex which enhances surface area increase, a process that is needed for cytokinesis itself [22,24].
Blebbing and embryogenesis: During embryonic development in different organisms, primordial germ cells (PGCs) first undergo different kinds of modifications [114] before leaving their site of specification and migrate to a distant site to meet the gonad where differentiation into eggs and sperms takes place [85]. These cells migrate by polarized bleb formation mediated by actomyosin contraction that is dependent on calcium accumulation at the leading edge [85]. In a similar vein, PGCs from Drosophila melanogaster and zebrafish migrate by blebbing in response to stromal cellderived factor 1, SDF-1 [24,85]. Motile progenitor cells of zebrafish during gastrulation use a combination of blebs and lamellipodia to migrate [115]. Recently, a form of blebbing called ‘stable blebbing’ was described in zebrafish embryos. The blebs in these cells were unique in that they exhibited exceptional migratory speed and persistence [116].
Blebbing and viral entry: There are now ample evidences that some viruses, rather than using micropinocytosis [117], can enter their host organisms using blebbing. For instance, it has been reported that kaposi’s sarcoma herpesvirus (KSHV) induced cytoskeletal reorganisation, and enter their target host, human dermal microvascular endothelial cells (HMVEC) by utilizing blebbing as a mechanism [118].
Conclusions
In general, the mode of migration a cell adopts at any given point is largely determined by the surrounding environment. While some cells migrate using single protrusion type [85,119], others exhibit plasticity, making interconvertibility of migration modes possible. Most cancer cells have defied protease inhibitors which were designed to target the conventional mesenchymal migration as a result of their ability to switch from lamellipodia to bleb formation (mesenchymalamoeboid transition, MAT) and this makes them to escape unperturbed through the pores of the ECM [15]. Over the past decades, extensive studies both at the cellular and molecular levels have been carried out to explain regulatory mechanisms underpinning plasma membrane blebbing, and these have broadened our current knowledge of the topic. These studies have impressive attempts at unraveling some molecules and signaling pathways contributing to cell blebbing. However, the actual cascade of events resulting from interactions of these molecules among themselves and with other pathways remains obscure and rudimentary, and thus requires detailed investigation. It has been shown that different cells do bleb, and that tumor cells mainly utilize it as an alternative migration mode in the extracellular matrix, however, knowledge of how blebbing translates into propelling a cell to migrate from one point to another in different environments is lacking. The guiding cues as well as how blebs orient themselves towards these cues during migration also require further investigation.
Acknowledgements
My thanks go to the cell migration laboratory in the molecular and cellular medicine division of the school of Life sciences at the University of Reading, United Kingdom for support and providing conducive environment for this work.
References
- Ponuwei GA (2016) A glimpse of the ERM proteins. Journal biomed sci 23: 35.
- Strychalski W, Guy RD (2016) Intracellular Pressure Dynamics in Blebbing Cells. Biophysical J 110: 1168-1179.
- Mierke CT (2015) Physical view on migration modes. Cell adhesion & migration 9: 367-379.
- Rana PS, Mudrak NJ, Lopez R, Lynn M, Kershner L, et al.(2017) Phase separation in necrotic cells. Biochem Biophys Res Commun 492: 300-303.
- Paluch EK, Raz E (2013) The role and regulation of blebs in cell migration. Curr Opin Cell Biol 25: 582-590.
- Tinevez JY, Schulze U, Salbreux G, Roensch J, Joanny JF, et al (2009) Role of cortical tension in bleb growth. Proc Natl Acad Sci USA 106: 18581-18586.
- Charras GT, Coughlin M, Mitchison TJ, Mahadevan L (2008) Life and times of a cellular bleb. Biophysical J 94: 1836-1853.
- Fackler OT, Grosse R (2008) Cell motility through plasma membrane blebbing. J Cell Biol 181: 879-884.
- Norman L, Sengupta K, Aranda Espinoza H (2011) Blebbing dynamics during endothelial cell spreading. Eur J Cell Biol 90: 37-48.
- Norman LL, Brugues J, Sengupta K, Sens P, Aranda Espinoza H (2010) Cell blebbing and membrane area homeostasis in spreading and retracting cells. Biophysical J 99: 1726-1733.
- Krause M, Gautreau A (2014) Steering cell migration: lamellipodium dynamics and the regulation of directional persistence. Nat Rev Mol Cell Biol 15: 577-590.
- Chang K, Baginski J, Hassan SF, Volin M, Shukla D, et al. (2016) Filopodia and Viruses: An Analysis of Membrane Processes in Entry Mechanisms. Front Microbiol 7: 300.
- Aramaki S, Mayanagi K, Jin M, Aoyama K, Yasunaga T (2016) Filopodia Formation by Cross-linking of F-actin with Fascin in Two Different Binding Manners. Cytoskeleton (Hoboken) 73: 365-374.
- Di Martino J, Henriet E, Ezzoukhry Z, Goetz JG, Moreau V, (2016) The microenvironment controls invadosome plasticity. J Cell Sci 129: 1759-1768.
- Wolf K, Mazo I, Leung H, Engelke K, von Andrian UH, et al. (2003) Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol 160: 267-277.
- Lorentzen A, Bamber J, Sadok A, Elson-Schwab I, Marshall CJ (2011) An ezrin-rich, rigid uropod like structure directs movement of amoeboid blebbing cells. J Cell Sci 124: 1256-1267.
- Hogue MJ (1919) The Effect of Hypotonic and Hypertonic Solutions on Fibroblasts of the Embryonic Chick Heart in Vitro. J Exp Med 30: 617-648.
- Boss J (1955) Mitosis in cultures of newt tissues. IV. The cell surface in late anaphase and the movements of ribonucleoprotein. Exp cell res 8: 181-187.
- Kubota HY (1981) Creeping locomotion of the endodermal cells dissociated from gastrulae of the Japanese newt, Cynops pyrrhogaster. Exp cell res 133: 137-148.
- Trinkaus JP (1973) Surface activity and locomotion of Fundulus deep cells during blastula and gastrula stages. Developmental biology 30: 69-103.
- Mills JC, Stone NL, Pittman RN (1999) Extranuclear apoptosis. The role of the cytoplasm in the execution phase. J Cell Biol 146: 703-708.
- Charras GT (2008) A short history of blebbing. J Microsc 231: 466-478.
- Barros LF, Kanaseki T, Sabirov R, Morishima S, Castro J, et al. (2003) Apoptotic and necrotic blebs in epithelial cells display similar neck diameters but different kinase dependency. Cell Death Differ 10: 687-697.
- Charras G, Paluch E (2008) Blebs lead the way: how to migrate without lamellipodia. Nat Rev Mol Cell Biol 9: 730-736.
- Goudarzi M, Banisch TU, Mobin MB, Maghelli N, Tarbashevich K, et al. (2012) Identification and regulation of a molecular module for bleb-based cell motility. Developmental cell 23: 210-218.
- Paluch E, Piel M, Prost J, Bornens M, Sykes C (2005) Cortical actomyosin breakage triggers shape oscillations in cells and cell fragments. Biophysical J 89: 724-733.
- Pollard TD, Borisy GG (2003) Cellular motility driven by assembly and disassembly of actin filaments. Cell 112: 453-465.
- Strychalski W, Guy RD (2013) A computational model of bleb formation. Math Med Biol 30: 115-130.
- Spangler EJ, Harvey CW, Revalee JD, Kumar PB, Laradji M (2011) Computer simulation of cytoskeleton-induced blebbing in lipid membranes. Physical review E, Statistical, nonlinear, and soft matter physics 84: 051906.
- Ikenouchi J, Aoki K (2016) Membrane bleb : a seesaw game of two small GTPases. Small GTPases 8: 85-89.
- Tozluoglu M, Mao Y, Bates PA, Sahai E (2015) Cost-benefit analysis of the mechanisms that enable migrating cells to sustain motility upon changes in matrix environments. J R Soc Interface 12: 1355.
- Charras GT, Hu CK, Coughlin M, Mitchison TJ (2006) Reassembly of contractile actin cortex in cell blebs. J Cell Biol 175: 477-490.
- Ponuwei GA, Dash PR (2016) Bleb Formation in Human Fibrosarcoma HT1080 Cancer Cell Line Is Positively Regulated by the Lipid Signalling Phospholipase D2 (PLD2). Achiev Life Sci 10: 125-135.
- Bovellan M, Fritzsche M, Stevens C, Charras G (2010) Death-associated protein kinase (DAPK) and signal transduction: blebbing in programmed cell death. The FEBS J 277: 58-65.
- Wang Y, Litvinov RI, Chen X, Bach TL, Lian L, et al. (2008) Loss of PIP5KIgamma, unlike other PIP5KI isoforms, impairs the integrity of the membrane cytoskeleton in murine megakaryocytes. J Clin Invest 118: 812-819.
- Diz Munoz A, Krieg M, Bergert M, Ibarlucea Benitez I, Muller DJ, et al. (2010) Control of directed cell migration in vivo by membrane-to-cortex attachment. PLoS biology 8: e1000544.
- Schepis A, Sepich D, Nelson WJ (2012) alphaE-catenin regulates cell-cell adhesion and membrane blebbing during zebrafish epiboly. Development 139: 537-546.
- Yanase Y, Hide I, Mihara S, Shirai Y, Saito N, et al. (2011) A critical role of conventional protein kinase C in morphological changes of rodent mast cells. Immunology cell bio 89: 149-159.
- Petrie RJ, Yamada KM (2012) At the leading edge of three-dimensional cell migration. Journal of cell science 125: 5917-5926.
- Svitkina TM, Bulanova EA, Chaga OY, Vignjevic DM, Kojima S, et al. (2003) Mechanism of filopodia initiation by reorganization of a dendritic network. J Cell Biol 160: 409-421.
- Bray D, White JG (1988) Cortical flow in animal cells. Science 239: 883-888.
- Ridley AJ (2011) Life at the leading edge. Cell 145: 1012-1022.
- Etienne Manneville S, Hall A (2002) Rho GTPases in cell biology. Nature 420: 629-635.
- Charras GT, Yarrow JC, Horton MA, Mahadevan L, Mitchison TJ (2005) Non-equilibration of hydrostatic pressure in blebbing cells. Nature 435: 365-369.
- Sanz Moreno V, Gadea G, Ahn J, Paterson H, Marra P, et al. (2008) Rac activation and inactivation control plasticity of tumor cell movement. Cell 135: 510-523.
- Yamazaki D, Kurisu S, Takenawa T (2009) Involvement of Rac and Rho signaling in cancer cell motility in 3D substrates. Oncogene 28: 1570-1583.
- Sahai E, Marshall CJ (2003) Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nature cell bio 5: 711-719.
- Wilkinson S, Paterson HF, Marshall CJ (2005) Cdc42-MRCK and Rho-ROCK signaling cooperate in myosin phosphorylation and cell invasion. Nature cell bio 7: 255-261.
- Jaffe AB, Hall A (2005) Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 21: 247-269.
- Sanz Moreno V, Marshall CJ (2010) The plasticity of cytoskeletal dynamics underlying neoplastic cell migration. Curr Opin Cell Biol 22: 690-696.
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A (1992) The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70: 401-410.
- Chen Z, Borek D, Padrick SB, Gomez TS, Metlagel Z, et al. (2010) Structure and control of the actin regulatory WAVE complex. Nature 468: 533-538.
- Gadea G, Sanz Moreno V, Self A, Godi A, Marshall CJ (2008) DOCK10-mediated Cdc42 activation is necessary for amoeboid invasion of melanoma cells. Current biology 18: 1456-1465.
- Clark EA, Golub TR, Lander ES, Hynes RO (2000) Genomic analysis of metastasis reveals an essential role for RhoC. Nature 406: 532-535.
- Stoletov K, Montel V, Lester RD, Gonias SL, Klemke R (2007) High-resolution imaging of the dynamic tumor cell vascular interface in transparent zebrafish. Proc Natl Acad Sci USA 104: 17406-17411.
- Truebestein L, Elsner DJ, Fuchs E, Leonard TA (2015) A molecular ruler regulates cytoskeletal remodelling by the Rho kinases. Nat Commun 6: 10029.
- Vega FM, Ridley AJ (2016) The RhoB small GTPase in physiology and disease. Small GTPases 1: 1-10.
- Jacobelli J, Bennett FC, Pandurangi P, Tooley AJ, Krummel MF (2009) Myosin IIA and ICAM 1 regulate the interchange between two distinct modes of T cell migration. J Immunol 182: 2041-2050.
- Takesono A, Heasman SJ, Wojciak Stothard B, Garg R, Ridley AJ (2010) Microtubules regulate migratory polarity through Rho/ROCK signaling in T cells. PloS one 5: e8774.
- Berton S, Belletti B, Wolf K, Canzonieri V, Lovat F, et al. (2009) The tumor suppressor functions of p27(kip1) include control of the mesenchymal/amoeboid transition. Mol cellular bio 29: 5031-5045.
- Ohta Y, Hartwig JH, Stossel TP (2006) FilGAP, a Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodelling. Nat Cell Biol 8: 803-814.
- Saito K, Ozawa Y, Hibino K, Ohta Y (2012) FilGAP, a Rho/Rho-associated protein kinase-regulated GTPase-activating protein for Rac, controls tumor cell migration. Mol bio cell 23: 4739-4750.
- Kawaguchi K, Saito K, Asami H, Ohta Y (2014) ADP ribosylation factor 6 (Arf6) acts through FilGAP protein to down-regulate Rac protein and regulates plasma membrane blebbing. J Biol Chem 289: 9675-9682.
- Bialik S, Kimchi A (2006) The death-associated protein kinases: structure, function, and beyond. Annu Rev Biochem 75: 189-210.
- Cohen O, Inbal B, Kissil JL, Raveh T, Berissi H, et al. (1999) DAP-kinase participates in TNF-alpha- and Fas-induced apoptosis and its function requires the death domain. J Cell Biol 146: 141-148.
- Bialik S, Bresnick AR, Kimchi A (2004) DAP-kinase-mediated morphological changes are localization dependent and involve myosin-II phosphorylation. Cell Death Differ 11: 631-644.
- Kuo JC, Lin JR, Staddon JM, Hosoya H, Chen RH (2003) Uncoordinated regulation of stress fibers and focal adhesions by DAP kinase. J Cell Sci 116: 4777-4790.
- Houle F, Poirier A, Dumaresq J, Huot J (2007) DAP kinase mediates the phosphorylation of tropomyosin-1 downstream of the ERK pathway, which regulates the formation of stress fibers in response to oxidative stress. J Cell Sci 120: 3666-3677.
- Yap AS, Kovacs EM (2003) Direct cadherin-activated cell signaling: a view from the plasma membrane. J Cell Biol 160: 11-16.
- Zhao S, Liao H, Ao M, Wu L, Zhang X, et al. (2014) Fixation-induced cell blebbing on spread cells inversely correlates with phosphatidylinositol 4,5-bisphosphate level in the plasma membrane. FEBS open bio 4: 190-199.
- Sugiyama T, Pramanik MK, Yumura S (2015) Microtubule Mediated Inositol Lipid Signaling Plays Critical Roles in Regulation of Blebbing. PloS one 10: e0137032.
- Panupinthu N, Zhao L, Possmayer F, Ke HZ, Sims SM, et al. (2007) P2X7 nucleotide receptors mediate blebbing in osteoblasts through a pathway involving lysophosphatidic acid. J Biol Chem 282: 3403-3412.
- Franca Koh J, Devreotes PN (2004) Moving forward: mechanisms of chemoattractant gradient sensing. Physiology (Bethesda) 19: 300-308.
- Sheetz MP, Sable JE, Dobereiner HG (2006) Continuous membrane cytoskeleton adhesion requires continuous accommodation to lipid and cytoskeleton dynamics. Annu Rev Biophys Biomol Struct 35: 417-434.
- Eisenmann KM, Harris ES, Kitchen SM, Holman HA, Higgs HN, et al. (2007) Dia-interacting protein modulates formin-mediated actin assembly at the cell cortex. Current bio 17: 579-591.
- Hannemann S, Madrid R, Stastna J, Kitzing T, Gasteier J, et al (2008) The Diaphanous-related Formin FHOD1 associates with ROCK1 and promotes Src-dependent plasma membrane blebbing. J Biol Chem 283: 27891-27903.
- Han Y, Eppinger E, Schuster IG, Weigand LU, Liang X, et al. (2009) Formin like 1 (FMNL1) is regulated by N-terminal myristoylation and induces polarized membrane blebbing. J Biol Chem 284: 33409-33417.
- Laser Azogui A, Diamant Levi T, Israeli S, Roytman Y, Tsarfaty I (2014) Met induced membrane blebbing leads to amoeboid cell motility and invasion. Oncogene 33: 1788-1798.
- Jia Z, Vadnais J, Lu ML, Noel J, Nabi IR (2006) Rho/ROCK-dependent pseudopodial protrusion and cellular blebbing are regulated by p38 MAPK in tumour cells exhibiting autocrine c-Met activation. Biology of the cell / under the auspices of the European Cell Biology Organization 98: 337-351.
- Maulik G, Kijima T, Ma PC, Ghosh SK, Lin J, et al. (2002) Modulation of the c-Met/hepatocyte growth factor pathway in small cell lung cancer. Clinic canc res 8: 620-627.
- Meshki J, Douglas SD, Lai JP, Schwartz L, Kilpatrick LE, et al. (2009) Neurokinin 1 receptor mediates membrane blebbing in HEK293 cells through a Rho/Rho-associated coiled-coil kinase-dependent mechanism. J Biol Chem 284: 9280-9289.
- Meshki J, Douglas SD, Hu M, Leeman SE, Tuluc F (2011) Substance P induces rapid and transient membrane blebbing in U373MG cells in a p21-activated kinase-dependent manner. PloS one 6: e25332.
- Weidinger G, Stebler J, Slanchev K, Dumstrei K, Wise C, et al. (2003) Dead end, a novel vertebrate germ plasm component, is required for zebrafish primordial germ cell migration and survival. Current bio 13: 1429-1434.
- Tyson RA, Zatulovskiy E, Kay RR, Bretschneider T (2014) How blebs and pseudopods cooperate during chemotaxis. Proc Natl Acad Sci USA 111: 11703-11708.
- Blaser H, Reichman Fried M, Castanon I, Dumstrei K, Marlow FL, et al. (2006) Migration of zebrafish primordial germ cells: a role for myosin contraction and cytoplasmic flow. Developmental cell 11: 613-627.
- Gutjahr MC, Rossy J, Niggli V (2005) Role of Rho, Rac, and Rho-kinase in phosphorylation of myosin light chain, development of polarity, and spontaneous migration of Walker 256 carcinosarcoma cells. Experimental cell res 308: 422-438.
- Rossy J, Gutjahr MC, Blaser N, Schlicht D, Niggli V (2007) Ezrin/moesin in motile Walker 256 carcinosarcoma cells: signal-dependent relocalization and role in migration. Experimental cell res 313: 1106-1120.
- Paluch E, Sykes C, Prost J, Bornens M (2006) Dynamic modes of the cortical actomyosin gel during cell locomotion and division. Trends Cell Biol 16: 5-10.
- Stockem W, Hoffmann HU, Gawlitta W (1982) Spatial organization and fine structure of the cortical filament layer in normal locomoting Amoeba proteus. Cell Tissue Res 221: 505-519.
- Pinner S, Sahai E (2008) PDK1 regulates cancer cell motility by antagonising inhibition of ROCK1 by RhoE. Nat Cell Biol 10: 127-137.
- Grebecki A, Grebecka L, Wasik A (2001) Minipodia and rosette contacts are adhesive organelles present in free-living amoebae. Cell bio international 25: 1279-1283.
- Sroka J, von Gunten M, Dunn GA, Keller HU (2002) Phenotype modulation in non-adherent and adherent sublines of Walker carcinosarcoma cells: the role of cell-substratum contacts and microtubules in controlling cell shape, locomotion and cytoskeletal structure. Int J Biochem Cell Biol 34: 882-899.
- Malawista SE, de Boisfleury Chevance A, Boxer LA (2000) Random locomotion and chemotaxis of human blood polymorphonuclear leukocytes from a patient with leukocyte adhesion deficiency-1: normal displacement in close quarters via chimneying. Cell motility cytoske 46: 183-189.
- Kardash E, Reichman Fried M, Maitre JL, Boldajipour B, Papusheva E, et al. (2010) A role for Rho GTPases and cell-cell adhesion in single-cell motility in vivo. Nat cell bio 12: 47-53.
- Bereiter Hahn J, Luck M, Miebach T, Stelzer HK, Voth M (1990) Spreading of trypsinized cells: cytoskeletal dynamics and energy requirements. J cell sci 96: 171-188.
- Wickman GR, Julian L, Mardilovich K, Schumacher S, Munro J, et al (2013) Blebs produced by actin-myosin contraction during apoptosis release damage associated molecular pattern proteins before secondary necrosis occurs. Cell Death Differ 20: 1293-1305.
- Croft DR, Coleman ML, Li S, Robertson D, Sullivan T, et al. (2005) Actin-myosin-based contraction is responsible for apoptotic nuclear disintegration. J Cell Biol 168: 245-255.
- Lowe SW, Lin AW (2000) Apoptosis in cancer. Carcinogenesis 21: 485-495.
- Wickman G, Julian L, Olson MF (2012) How apoptotic cells aid in the removal of their own cold dead bodies. Cell Death Differ 19: 735-742.
- Orlando KA, Stone NL, Pittman RN (2006) Rho kinase regulates fragmentation and phagocytosis of apoptotic cells. Experimental cell res 312: 5-15.
- Babiychuk EB, Monastyrskaya K, Potez S, Draeger A (2011) Blebbing confers resistance against cell lysis. Cell Death Differ 18: 80-89.
- Segundo C, Medina F, Rodriguez C, Martinez Palencia R, Leyva Cobian F, et al. (1999) Surface molecule loss and bleb formation by human germinal center B cells undergoing apoptosis: role of apoptotic blebs in monocyte chemotaxis. Blood 94: 1012-1020.
- Cunningham CC (1995) Actin polymerization and intracellular solvent flow in cell surface blebbing. J Cell Biol 129: 1589-1599.
- McGrath JL (2007) Cell spreading: the power to simplify. Current bio 17: 357-358.
- Zatulovskiy E, Kay RR (2016) Chemotactic Blebbing in Dictyostelium Cells. Methods Mol Biol 1407: 97-105.
- Zatulovskiy E, Tyson R, Bretschneider T, Kay RR (2014) Bleb-driven chemotaxis of Dictyostelium cells. The Journal of cell biology 204: 1027-1044.
- Yanase Y, Carvou N, Frohman MA, Cockcroft S (2010) Reversible bleb formation in mast cells stimulated with antigen is Ca2+/calmodulin-dependent and bleb size is regulated by ARF6. Biochem J 425: 179-193.
- Khajah MA, Luqmani YA (2015) Involvement of Membrane Blebbing in Immunological Disorders and Cancer. Medical principles and practice : inter J international journal of the Kuwait University Kuwait University 2: 18-27.
- Khajah MA, Mathew PM, Alam-Eldin NS, Luqmani YA (2015) Bleb formation is induced by alkaline but not acidic pH in estrogen receptor silenced breast cancer cells. Int J Oncol 46: 1685-1698.
- Friedl P, Wolf K (2003) Tumour cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 3: 362-374.
- Derivery E, Fink J, Martin D, Houdusse A, Piel M, et al. (2008) Free Brick1 is a trimeric precursor in the assembly of a functional wave complex. PloS one 3: e2462.
- Burton K, Taylor DL (1997) Traction forces of cytokinesis measured with optically modified elastic substrata. Nature 385: 450-454.
- Boucrot E, Kirchhausen T (2007) Endosomal recycling controls plasma membrane area during mitosis. Proceedings of the National Academy of Sciences of the United States of America 104: 7939-7944.
- Blaser H, Eisenbeiss S, Neumann M, Reichman Fried M, Thisse B, et al. (2005) Transition from non-motile behaviour to directed migration during early PGC development in zebrafish. J cell sci 118: 4027-4038.
- Row RH, Maitre JL, Martin BL, Stockinger P, Heisenberg CP, et al. (2011) Completion of the epithelial to mesenchymal transition in zebrafish mesoderm requires Spadetail. Developmental biology 354: 102-110.
- Ruprecht V, Wieser S, Callan Jones A, Smutny M, Morita H, et al. (2015) Cortical contractility triggers a stochastic switch to fast amoeboid cell motility. Cell 160: 673-685.
- Koivusalo M, Welch C, Hayashi H, Scott CC, Kim M, et al. (2010) Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J Cell Biol 188: 547-563.
- Veettil MV, Bandyopadhyay C, Dutta D, Chandran B (2014) Interaction of KSHV with host cell surface receptors and cell entry. Viruses 6: 4024-4046.
- Svitkina TM, Verkhovsky AB, McQuade KM, Borisy GG (1997) Analysis of the actin-myosin II system in fish epidermal keratocytes: mechanism of cell body translocation. J Cell Biol 139: 397-415.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences