ISSN : 2321-2748
American Journal of Phytomedicine and Clinical Therapeutics
The use of herbal products during pregnancy: which is the risk perception
1Department of Physiology and Pharmacology, Sapienza University of Rome P. le A. Moro, 5 - 00185 – Rome, Italy
2Department of Behavioral Neuroscience, Oregon Health & Science University; VA Portland Health Care System, Research and Development Service (R&D39), 3710 SW Veterans Hospital Road; Portland, OR 97239, USA
3Maternal-Child Department and Urological Sciences, Sapienza University of Rome, Viale del Policlinico 155, 00161 Rome – Italy
- *Corresponding Author:
- Olta Allkanjari
Department of Physiology and Pharmacology
Sapienza University of Rome
P. le A. Moro, 5 - 00185 – Rome
Tel: + 00390649912904
E-mail: olta.allkanjari@uniroma1.it
Received Date: October 30, 2020; Accepted Date: December 28, 2020; Published Date: January 05, 2021
Citation: Vitalone A, Allkanjari O, Durazzi F, Guizzetti M, Aleandri V (2021) The use of herbal products during pregnancy: which is the risk perception?. Am J Phytomed Clin Ther Vol.9 No.1:1.
Abstract
The aim of the study was to assess women habits during pregnancy, the prevalence of use of herbal products and the awareness about the related risk of adverse reactions. A total of 279 interviewees, at Polyclinic Umberto I (Rome, Italy), completed the questionnaire that examined descriptive and analytical aspects about herbal products. The majority quit smoking and drinking alcohol, but consumed regularly herbal products, often associated with drug therapies. Pregnant women considered herbal products less dangerous than other medications, even if some dermatological and gastrointestinal adverse reactions were reported. Gynecologist and herbalist represent the primary source of information about herbal products, while the most common place of purchase is the pharmacy. Pregnant women appear mindful about the risks of smoking, alcohol and medications. On the contrary, their knowledge about the risk/ benefit profile of herbal products is limited. Therefore, information strategies and surveillance programs about the safety of herbal products should be implemented in the context of public health.
Keywords
Herbal dietary supplements; Pregnancy; Safety; Herbal products; Adverse reactions; Women; Phytomedicine
Abbreviations
HPs: Herbal Products; CYP: Cytochrome
Introduction
Women, especially during pregnancy, seem to be common users of herbal products (HPs) to prevent/reduce the risk of diseases or to improve the state of well-being. Pregnant women belong to one of the susceptible population subgroups exposed to more risk factors; therefore, attention monitoring and observational studies are constantly required. In various countries, contrasting results exist about the knowledge on the safety profile of HPs, while they are conscious about the possible risk of exposure to other xenobiotics (medications, etc.) [1-3]. In particular, Iranian pregnant women mostly do not inform the physician about the herbal supplementation [4], whereas Australian women search information from reputable sources (e.g. healthcare professionals, government or hospital websites) in order to consume or not an herbal product [5]. In Italy, pregnant women appear even more inclined to take HPs than women of other countries [6]. They probably minimize the related risk of adverse reactions, even though some of these products are specifically contraindicated during pregnancy (anthranoids) [7]. Nevertheless, the use of HPs during pregnancy is not fully studied [2].
Unlike herbal medicinal products that are authorized by the Italian Medicines Agency and/or European Medicines Authority [8], herbal dietary supplements are marketed after notification to the Italian Ministry of Health, with no need of efficacy and safety (evidence-based) proofs. Moreover, herbal medicinal products are controlled by the National Pharmacovigilance Network, which is directly coordinated by the Italian Medicines Agency. On the other hand, the collection of suspected adverse reactions due to cosmetics are managed by the Italian Ministry of Health, whereas adverse reactions related to other products (herbal supplements, vitamins, etc.) are subject of a separate system (Phytosurveillance) coordinated by the Italian Institute of Health [8]. This multiplicity of definitions/regulations of the various products could lead to extreme confusion among consumers. In addition, often overestimated claims of effectiveness in treating a range of conditions are made by HP manufacturer companies, increasing consumer/healthcare professionals’ decision-making difficulties [9].
Even though it is well known that alcohol use during pregnancy can cause developmental disorders and there is substantial awareness among pregnant women about the risks to the fetus of drinking alcohol during gestation, there are many studies that aim to estimate the prevalence of drinking and to enhance prevention strategies in order to further increase the awareness of the public [10]. Likewise, smoking is another well-established risk factor for pregnancy complications; prevention efforts and global studies are continuously made, to increase the perception of the population, especially of the pregnant women [11]. The intake of herbal products in pregnancy does not have the same attention. Even though the use of HPs is diffused during pregnancy, there is a lack of clinical data reporting the frequency of use and supporting their risk/benefit profile [12].
Hence, the aim of the present study was to assess women habits during pregnancy, the prevalence of use of HPs and the awareness of pregnant women about their safety and effectiveness.
Methods
The methods used in this survey have been previously published [1]. Some details are given below. We consider HPs all products (herbal dietary supplements, cosmetics, homeopathic remedies, and herbal medicinal products) containing an herbal substance [1].
A mono-centric observational survey has been conducted retrospectively at the Polyclinic Umberto I in Rome for a 12-month period (from June 2017 to June 2018). Umberto I is one of the largest public hospitals in Italy, which serves a heterogeneous population of patients representative of different ethnicities and socio-economic status. Pregnant women (280) undergoing gynaecological examination, in different gestational stages, were randomly interviewed. Qualified healthcare personnel collected the information twice a week, through a structured face to face questionnaire administered to each woman. The survey was organized in 20 questions divided in descriptive (age, nationality, gestation week, previous pregnancies, level of education, occupation, smoking and drinking habits, favourite foods,) and analytical (characteristics of HPs used, place of purchase of herbal products, main source of information on their safety and effectiveness, concomitant therapies, adverse reactions potentially due to HPs) questions in Italian language, previously published and validated [1,13]. Main outcome measures consisted in herbal products and other medications taken during pregnancy and consumers awareness on their safety and effectiveness. At each interview, according to the Declaration of Helsinki, the women gave informed consent after receiving a description of the study, and the privacy protection was applied during the collection of data. Using the Office Excel software, an identifying number was assigned to each questionnaire in order to ensure anonymity.
In line with the Italian guidelines for the classification and conduction of observational studies on drugs (Official Gazette of the Italian Republic, GU n. 76 of 31-3-2008), the study has been notified/approved by the ethical committee of the institution where the survey was carried out (notification/registration of the protocol approved 12/22/2016).
Results
Among 280 questionnaires provided to pregnant women, two hundred seventy-nine (279) women joined the project, with a mean age 32.4 ± 0.34 years; they were on average at the thirtythird ± 0.44 week of pregnancy.
Descriptive characteristics of the interviewees
50.5% of the women in this study (n=141) did not have previous pregnancies, whereas 49.5% (n=138) of women were multiparous. Among multiparous responders (n=137), 62.0% (n=85) of women had previously one pregnancy, 23.4% (n=32) of women had two pregnancies, 8.0% (n=11) of women had three pregnancies, and 6.5% (n=9) of women had experienced more than three pregnancies.
Referred to questionnaires, eight women did not answer to this specific question. Among 171 women who answered to the question, 80.1% (n=137) of participants were from Italy while 19.9% (n=34) of them were from foreign countries, in particular, 6.4% (n=11) were from Romania, 1.8% (n=3 were from Ecuador, 1.8% (n=3) were from Poland, 1.2% (n=2) were from Germany, 1.2% (n=2) were from Peru, 1.2% (n=2) were from Brazil, 1.2% (n=2) were from Albania, 1.2% (n=2) were from Bangladesh. Women from other countries (such as China, Russia, etc.) represented 4.1% (n=7) of the interviewees.
The majority of the pregnant women interviewed had a high school diploma, were employed, neither smoked nor consumed alcohol. Meanwhile, 84.4% (n=232) of them were daily consumers of HPs, 15.6% (n=43) of the women had never used these products and four of them did not answer. In particular, 85.5% (n=230) used HPs even during pregnancy; ten women did not answer this specific question. A more detailed summary of the descriptive characteristics of the women who responded to the questionnaire is reported in Table 1.
| Characteristics | Response rate for each question |
|---|---|
| Level of education | - Secondary school certificate: 19.6% (54/276) |
| - High school: 54.3% (150/276) | |
| - College degree: 26.1% (72/276) | |
| Occupation | - Clerk: 29.9% (83/278) |
| - Self-employed (e.g. beautician, hairdresser, saleswoman, secretary, seamstress): 14.7% (41/278) | |
| - Worker: 10.8% (30/278) | |
| - Freelance (e.g., architect, lawyer, accountant, pharmacist): 9.7% (27/278) | |
| - Housewife: 20.8% (58/278) | |
| - Teacher: 5.8% (16/278) | |
| - Student: 3.6% (10/278) | |
| - Unemployed or other: 4.6% (13/278) | |
| Favorite food | - Carbohydrates (pasta, bread): 31.0% (77/248) |
| - Vegetable (salad, spinach, eggplant etc.): 25.8% (64) | |
| - Fruit (grapes, apples, etc.): 21.4% (53) | |
| - Meat (red meat, chicken, etc.): 18.1% (45) | |
| - Fish (shrimp, tuna, etc.): 3.2% (8) | |
| - Other (dairy products): 0.4% (1) | |
| Smoking | - Never smoker: 50.9% (139/273) |
| - Previous smoker: 35.5% (97/273) | |
| - Current smoker: 13.6% (37/273) | |
| Alcohol use | - No, never: 85.4% (217/254) |
| - Yes, occasionally (less than three glasses per week) : 12.6% (32/254) | |
| - Yes, quite frequently (3-14 glasses of alcohol /week): 2.0% (5/254) |
Table 1: Descriptive characteristics of pregnant women who responded to the questionnaire (n=279).
Characteristics of herbal products used by pregnant women
Among interviewees, the category of products mostly used was that of cosmetics (all of these contain at least one plant extract), followed by food supplements (i.e., vitamins and/or minerals, without herbal ingredients), herbal dietary supplements, homeopa thic medicines and herbal medicinal products. The specific characteristics/composition of products used by pregnant women is reported in Table 2. These products were usually used for almost the entire period of gestation (157.2 ± 4.8 days), for reasons primary due to pregnancy (86.4%; n=203), including dermatologic problems, gastrointestinal discomforts, mild vascular disease, etc. (data not shown).
| Product | Most representative ingredients (where specified). |
|---|---|
| Cosmetics (73.6%; n=190) | - Prunus amygdalus var. dulcis - almond oil (66.8%; n=127); |
| - Multi-herbal ingredients, including rice oil in association with tapioca starch, bran oil, Pisum sativum - pea (18.9%; n=36); | |
| - Lawsonia inermis - henna (4.2%; n=8) | |
| - Calendula (2.6%; n=5); | |
| - Aloe gel (2.6%; n=5); | |
| - Others (5.8%; n=11) | |
| Herbal dietary supplements (42.6%; n=110) | - Foeniculum vulgaris - fennel (32.7%; n=36); |
| - Chamomilla recutita - chamomile (30%; n=33) | |
| - Green tea (7.3%; n=8); | |
| - Black tea (5.5%; n=6) | |
| - Licorice (4.5%; n=5); | |
| - Others (unspecified multiple-ingredients herbal teas – 24.5%; n=27) | |
| Food supplements (95.3%; n=246) | - Multivitamin supplements, including those containing essential fatty acids, lutein, etc. (11.8%; n=29) |
| - Propolis (6.1%; n=15) | |
| - Others (non-herbal, iron, multi minerals, etc. – 9.3%, n=23) | |
| Homeopathic medicines (14%; n=36) | - Arnica (8.3%; n=3) |
| - Multi-ingredient syrup (5.6%; n=2) | |
| Herbal medicinal products (11.7%; n=30) | - Anthraquinones (26.7%; n=8) |
| - Centella (6.7%; n=2) | |
| - Valerian (6.7%; n=2); |
Table 2: Characteristics of products used by pregnant women (n=258)*.
Analytical characteristics of HPs used by pregnant women
The analytical aspects of the interview (e.g., source of information, place of purchase, use of medications) are described in Table 3.
| Analytical aspects | Response rate for each question* |
|---|---|
| Primary source of information on herbal products | - Gynecologist: 57.6% (147/255) |
| - Herbalist: 29.4% (75/255) | |
| - General practitioner: 20.8% (53/255) | |
| - Pharmacist: 14.9% (38/255) | |
| - Personal experience: 12.8% (33/255) | |
| - Friends: 9.0% (23/255) | |
| - Pediatrician: 7.1% (18/255) | |
| - Internet websites: 7.1% (18/255) | |
| Principal place of purchase of herbal products | - Pharmacy: 59.7% (154/258) |
| - Herbalist’s shops: 37.6% (97/258) - Supermarket: 22.1% (57/258) |
|
| - Perfumery: 7.8% (20/258) | |
| - Internet: 0.4% (1/258) | |
| - Catalogues: 0.4% (1/258). | |
| Use of other medications | - Yes: 66.0% (167/253) |
| - No: 34.0% (86/253) | |
| Major target system of other therapies | - Hematopoietic system: 46.7% (78/166) |
| - Endocrine system: 41.3% (69/166) | |
| - Cardiovascular system: 28.7% (48/166) | |
| - Gastrointestinal apparatus: 15.5% (26/166) | |
| - Anti-infective: 9.6% (16/166) | |
| - Nervous system: 3.0% (5/166) | |
| - Other (e.g., musculoskeletal apparatus, respiratory system): 3.6% (6/166) | |
| Adverse reactions potentially due to herbal products | - No: 92.5% (233/252) |
| - Yes: 7.5% (19/252) |
Table 3: Analytical aspects about HPs used by pregnant women who responded to the questionnaire.
HPs were considered as always safe in 65.2% (n=182) of the cases, while medications in only 4.7% (n=13). Only 7.1% (n=20) of the pregnant women considered HPs always harmful, while 14.7% (n=41) of them considered HPs dangerous only during the first trimester. In contrast, the use of medications is thought being always harmful in the 52.0% (n=145) of cases, and dangerous only during the first trimester in the 35.5% (n=99). Some women did not know the answer regarding the dangerousness of HP (12.9%, n=36) and of medications (7.9%, n=22).
Noteworthy, in the 66% of pregnant women, the use of herbal products was associated with other medicines. The drugs more used, were those for the hematopoietic system (e.g., Folina®, Prefolic®, Ferrograd®), specifically for red blood cells maturation and in the treatment of anemia and/or thrombophilia. Other prescribed drugs included those for: the endocrine system, such as prednisone (Deltacortene®), levothyroxine (Eutirox®), oxytocin (Syntocinon®, used to stimulate uterine contractions) etc.; cardiovascular system, as nifedipine (Adalat®, Clexane®); and gastrointestinal system, as sodium alginate associated with sodium bicarbonate (Gaviscon®), and an association of multiple salts (e.g., calcium carbonate, sodium carbonate, alginate and bicarbonate – Maalox RefluRAPID®) to treat stomach ache.
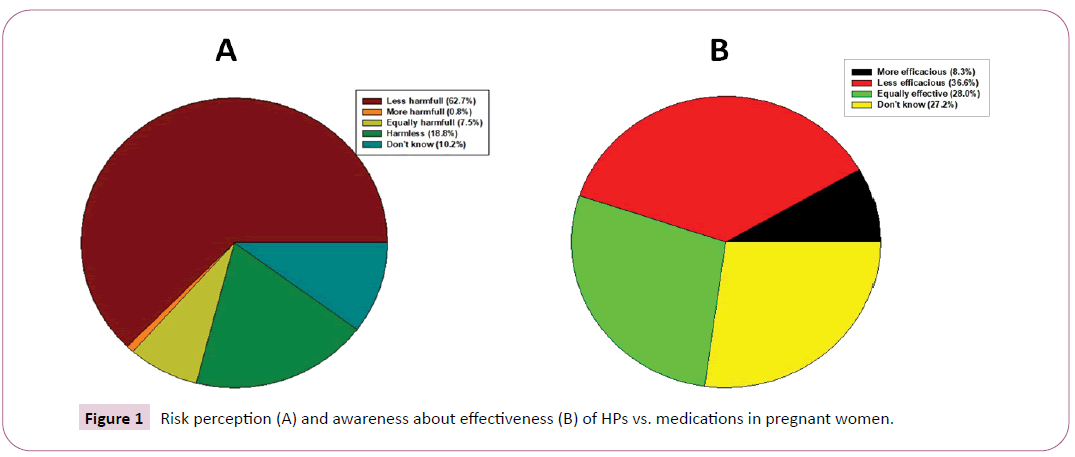
The risk perception and the awareness about effectiveness of HPs vs. medications are reported in Figure 1.
The interviewees considered HPs, less effective (36.6%; n=93) and less dangerous than medications (62.7%; n=160). However, 7.5% (n=19) of women have experienced adverse effects from HPs, concerning dermatological (21.1%; n=4), gastrointestinal (52.6%; n=10), or cardiovascular systems (5.3%; n=1). In particular, skin reactions that consisted in eczema, erythema, and pruritus were ascribed to almond oil usage; gastrointestinal adverse effects included hyperacidity, diarrhea, sickness, nausea and abdominal cramps were possibly due to anthrachinonic components of Aloe vera, Rheum palmatum, Cascara sagrada, Senna angustifolia. These adverse effects were reported when HPs were taken in combination with other medicinal plants. Cardiovascular reactions, mostly tachycardia, were ascribed to the use of Camelia sinensis (tea).
Discussion
This study highlights the highly prevalent use of HPs and the perception of the relative safety of HPs during pregnancy, in contrast to smoking, alcohol and drugs consumption, in a cohort of pregnant women. Although this is a monocentric survey, it provides information about women from different socioeconomic status and country of origin; women enrolled into the study were seen at the Umberto I Polyclinic, one of the biggest hospitals in Italy [1].
Though the use of herbal products during pregnancy is growing, the safety awareness remains a big problem. The marketing of herbal dietary supplements doesn’t need any evidence-based research [8], and the sale of these products in the internet evades regulations, contributing to an unconscious exposure of pregnant women. Recent data from different countries suggest herbal supplements during pregnancy to be a potential risk for children’s health [14,15]. In specific: Therefore, information about a proper use and the adverse effects of HPs is needed
In our study, the interviewed women appear aware of having to reduce alcohol consumption and smoking, to help preventing birth defects. On the contrary, from our results reported in Table 1, it comes out that the consumption of fish and dairy products are in lower percentage than it could be expected. Despite pregnant women are aware of the benefits of a healthy diet, it seems that some of these changes (e.g. reduced consume of fish, vegetables) are made in contrast with recommendations. Probably, the high percentage of women, who take multivitamin supplements and/or essential fatty acids, could explain (but not justify) their reduced fish consume.
Pregnant women are treated pharmacologically for diseases that are not often directly attributable to the pregnancy itself. Indeed, the use of drugs that occurs under medical supervision, regards ailments that affect hematopoietic (anemia, folate deficiency, thrombophilia, etc.), endocrine (e.g., immune thrombocytopenia, rheumatoid arthritis, systemic lupus erythematosus, maternal hypothyroidism), and cardiovascular systems (angina, hypertension, etc.). The women are very careful and selective in taking medicines that are extremely necessary; in particular, the iron replacement to treat hematopoietic disease, low-molecularweight heparins (LMWH-dalteparin, enoxaparin, etc.), folic acid supplementation, in order to ameliorate plasma volume, to counteract thrombogenic activities, and to prevent neurologic anomalies (e.g., anencephaly, encephalocele, spina bifida) [16,17]. Treatment of immune and endocrine systems pathologies consist in levothyroxine and corticosteroids (i.e., prednisone) therapies. Cardiovascular diseases are usually treated with a dihydropyridine calcium channel antagonistic (i.e., nifedipine, as a vasodilator agent with anti-anginal, antihypertensive, and tocolytic properties) [18].
In our study emerged that pregnant women are regular consumers of HPs (herbal dietary supplements, cosmetics, homeopathic remedies, herbal medicinal products), probably because of considering them of equal efficacy and safer than medications, or actually harmless. About half of interviewed women used herbal dietary supplements for pregnancyrelated problems (e.g., varicose legs, restlessness, insomnia, gastrointestinal problems) as fennel, chamomile, green and black tea, licorice, etc. Almost all of them took food supplements containing vitamins, fatty acids, lutein, propolis, etc. One third of pregnant women used cosmetic products to treat skin problems (e.g., irritations, hyperpigmentation, stretch marks) using aloe gel, calendula, pea extract, almond oil or to dye the hair using henna extract. It should be underlined that the therapeutic activity is a distinctive characteristic of the drugs, but often it is attributed to complementary medicines, including cosmetics and herbal dietary supplements. Moreover, almost one third of them used homeopathic (multi-ingredient syrups, arnica) and herbal medicinal products (anthraquinones, valerian, centella) revealing a poor and/or erroneous perception about their safety. Indeed some adverse reactions have been reported, consisting in eczema, erythema, and pruritus (ascribed to the use of almond oil); gastrointestinal adverse effects include hyperacidity, diarrhea, sickness, nausea and abdominal cramps possibly due to anthraquinone derivatives (Aloe vera, Cascara sagrada, Rheum palmatum, Senna angustifolia). Even if there are no reports of undesirable effects during pregnancy and on the foetus, experimental data concerning a genotoxic risk of several anthranoids (in particular, emodin and aloe-emodin) do already exist and for this reason these products are not recommended during pregnancy [7].
The possibility of adverse reactions due to HP is often underestimated. However, in Italy, from April 2002 to March 2017, more than 1500 reports of adverse reactions related to them have been collected [19]. Most of the reports could have been predicted because of the biological activities of the plants involved, in particularly the cardiovascular reactions characterized by tachycardia, related to Camelia sinensis (tea) [20]. In this case, the patient was also affected by cardiovascular disease (i.e., hypertension). We assume that although tachycardia might not be strictly related to its use, the green tea represent a potential risk factor of the above side effect [21]. The presence of confounding factors (i.e., concomitant pathologies, and/or medications) does not allow affirming precise conclusions.
In pregnant women, the possibility of herb-drug interactions could be translated in moderate to severe effects for the mother and fetus [22]. Various plants reported in our study, might interact with drugs, including green tea (inhibition of cytochrome P450 (CYP) 3A and P-glycoprotein [23], chamomile (inhibition of CYP1A2 and CYP3A4) [24], and licorice (induction of CYP3A and inhibition of P-glycoprotein) [25]. Moreover, there are significant evidences of CYP2D6 and CYP3A4 isoenzymes inhibition by Foeniculum vulgare extracts [26]. This means that if specific foods (e.g., green tea, fennel) are taken at the same time with other medications, an increase in bioavailability (that has been documented in in vitro studies) should be taken into account [27]. Obviously, we should also consider the lack of clinical pharmacokinetic studies that could explain these effects, as long as the levels and kinetics (absorption, distribution, metabolism, excretion) of plants used as foods, could be certainly different from those of in vitro experimental evidence.
The topical use of cosmetics, in particular of skin elasticizing products, which appears with high frequency in pregnant women, should be monitored. In this context, there are suggestions that associate almond oil abdomen application with premature myometrium contractions [2]. This event should be highlighted, even if the effect was probably related to the massage itself and not to the plant. Scientific literature data have reported a potential teratogenicity of Lawsonia inermis (henna) in animals [28]. In the present study the use of this plant among women interviewed is not very common and only topical.
Specialized healthcare professionals like gynecologists, herbalists, and general practitioners represent the primary source where the interviewed women took information/advice about herbal products. However, it is not always reassuring that physicians (gynecologists and general practitioners) could represent the first source of information for pregnant women, as in Italy, they do not have professional training in food-herbal supplements during their degree course [29]. Consequently, it could be difficult to provide unbiased safety advices to their patients about herbal products, if they don’t pursue postgraduate courses. On the other hand, the main place of purchase of herbal products is the pharmacy that represents a safe social-health source. Paradoxically, pharmacists are not valued by pregnant women for their professional expertise. Pharmacists themselves might underestimate the importance of their professional advices to pregnant women that seek information on HPs. Moreover, pregnant women purchase herbal products also at the supermarket and perfumery, places where there are no healthcare professionals; the information available to women in these places is advertising rather than science. The issue that arises from this “vicious cycle” of places of purchase and sources of information is the lack of a proper scientific guidance about HPs that would guide pregnant women to a correct use. This could be improved by enhancing professional competences. This study evidences the need for public health interventions through efforts consisting in accurate and comprehensive scientific information on HPs.
The limitations of our study were the following: 1. some women didn’t answer all the questions; 2. the product composition (and the identification of the herbal species) was not always accurate, as it was self-reported by the interviewed women; 3. We do not know the pregnancy outcome, as the place of delivery does not necessarily match with the hospital where women underwent to gynecological examination. The latter could lead to an underestimation of adverse reactions.
Conclusion
Pregnant women have a limited risk perception about HPs while they appear mindful about the risks of smoking, alcohol consumption and medications. Therefore, it is important to emphasize, paradoxically, that it is safer taking an old drug than a new herbal product. Moreover, as gynecologists and herbalists represent the primary source of information and pharmacy is the main place of purchase of HPs, the roles of these healthcare professionals in giving advice to pregnant women should be increased. As there is an increasing of HPs use, scientific data about their risk/benefit profile in pregnancy are needed. In order to prevent toxicological xenobiotic misuse, and to increase the consciousness about the safety of HP, more effective surveillance strategies for sensitive population subgroups like pregnant women, should be carried out by the Phytovigilance system.
Acknowledgements
We want to thank all women that have accepted to answer to the questionnaire of this survey, and the Enrico and Enrica Sovena Foundation for supporting this study.
References
- Aleandri V, Bertazzoni G, Romanzi D, Vetrano G, Durazzi F, et al. (2014) The Use of Herbal Products during Breastfeeding: A Study from a Public Italian Hospital. J Food Process Technol 5: 354.
- Facchinetti F, Pedrielli G, Benoni G, Joppi M, Verlato G, et al. (2012) Herbal supplements in pregnancy: unexpected results from a multicentre study. Hum Reprod 27: 3161-3167.
- Gilmartin CE, Vo-Tran TH, Leung L (2018) Complementary medicines in pregnancy: recommendations and information sources of healthcare professionals in Australia. Int J Clin Pharm 40: 421-427.
- Yazdi N, Salehi A, Vojoud M, Sharifi MH, Hoseinkhani A (2019) Use of complementary and alternative medicine in pregnant women: A cross-sectional survey in the south of Iran. J Integr Med 17: 392-395.
- Barnes LAJ, Barclay L, McCaffery K, Aslani P (2019) Factors influencing women’s decision-making regarding complementary medicine product use in pregnancy and lactation. BMC Pregnancy Childbirth 19: 280.
- Louik C, Gardiner P, Kelley K, Mitchell AA (2010) Use of herbal treatments in pregnancy. Am J Obstet Gynecol 202: 439.e1-e10.
- European Medicines Agency (2019) Doc. Ref. EMEA/HMPC/51869/2006 Corrigendum. Community herbal monograph on Cassia senna L and Cassia angustifolia Vahl, folium Accessed 19 December.
- Vitalone A, Menniti-Ippolito F, Raschetti R, Renda F, Tartaglia L, et al. (2012) Surveillance of suspected adverse reactions to herbal products used as laxatives. Eur J Clin Pharmacol 68: 231-238.
- Marra MV, Bailey RL (2018) Position of the Academy of Nutrition and Dietetics: Micronutrient Supplementation. J Acad Nutr Diet 118: 2162-2173.
- Popova S, Lange S, Probst C, Gmel G, Rehm J (2017) Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: A systematic review and meta-analysis. Lancet Glob Health 5: e290-e299.
- Lange S, Probst C, Rehm J, Popova S (2018) National, regional, and global prevalence of smoking during pregnancy in the general population: a systematic review and meta-analysis. Lancet Glob Health 6: e769-e776.
- Dante G, Pedrielli G, Annessi E, Facchinetti F (2013) Herb remedies during pregnancy: a systematic review of controlled clinical trials. J Matern Fetal Neonatal Med 26: 306-312.
- Lapi F, Vannacci A, Moschini M, Cipollini F, Morsuillo M, et al. (2010) Use, Attitudes and Knowledge of Complementary and Alternative Drugs (CADs) Among Pregnant Women: A Preliminary Survey in Tuscany. Evid Based Complement Alternat Med 7: 477-486.
- Ahmed M, Hwang JH, Choi S, Han D (2017) Safety classification of herbal medicines used among pregnant women in Asian countries: A systematic review. BMC Complement Altern Med 17: 489.
- Lewicka A, Szymański Ł, Rusiecka K, Kucza A, Jakubczyk A, et al. (2019) Supplementation of Plants with Immunomodulatory Properties during Pregnancy and Lactation-Maternal and Offspring Health Effects. Nutrients 11: 1958
- Sun D, McLeod A, Gandhi S, Malinowski AK, Sheata N (2017) Anemia in Pregnancy: A Pragmatic Approach. Obstet Gynecol Surv 72: 730-737.
- Skeith L (2017) Preventing venous thromboembolism during pregnancy and postpartum: crossing the thereshold. Hematology Am Soc Hematol Educ Program 2017: 160-167.
- Webster LM, Myers JE, Nelson-Piercy C, Harding K, Cruickshank JK, et al. (2017) Labetalol Versus Nifedipine as Antihypertensive Treatment for Chronic Hypertension in Pregnancy: A Randomized Controlled Trial. Hypertension 70: 915-922.
- Menniti-Ippolito F, Vitalone A, Da Cas R, Mazzanti G (2017) Suspected adverse reactions to herbal dietary supplements for weight loss collected by the Italian surveillance system on natural products. Drug Safety 40: 960.
- Vitalone A, Menniti-Ippolito F, Moro PA, Firenzuoli F, Raschetti R, et al. (2011) Suspected adverse reactions associated with herbal products used for weight loss: a case series reported to the Italian National Institute of Health. Eur J Clin Pharmacol 67: 215-224.
- Bedrood Z, Rameshrad M, Hosseinzadeh H.(2018) Toxicological effects of Camellia sinensis (green tea): A review Phytother Res 32: 1163-1180.
- McLay JS, Izzati N, Pallivalapila AR, Shetty A, Pande B, et al. (2017) Pregnancy, prescription medicines and the potential risk of herb-drug interactions: a cross-sectional survey. BMC Complement. Altern Med 17: 543.
- Werba JP, Misaka S, Giroli MG, Shimomura K, Amato M, et al. (2018)Update of green tea interactions with cardiovascular drugs and putative mechanisms. J Food Drug Anal 26: S72-S77.
- Gardiner P (2007) Complementary, holistic, and integrative and medicine: chamomile. Pediatr Rev 28: e16-e18.
- Feng X, Ding L, Qiu F (2015) Potential drug interactions associated with glycyrrhizin and glycyrrhetinic acid. Drug Metab Rev 47: 229-238.
- Langhammer AJ, Nilsen OG (2014) In vitro inhibition of human CYP1A2, CYP2D6, and CYP3A4 by six herbs commonly used in pregnancy. Phytother Res 28: 603-610.
- Sadati SN, Ardekani MR, Ebadi N, Yakhchali M, Dana AR, et al. (2016) Review of Scientific Evidence of Medicinal Convoy Plants in Traditional Persian Medicine. Pharmacogn Rev 10: 33-38.
- Jafarzadeh L, Seifi N, Shahinfard N, Sedighi, M, Kheiri S, et al. (2015) Antioxidant Activity and Teratogenicity Evaluation of Lawsonia Inermis in BALB/c Mice. J Clin Diagn Res 9: FF01-FF04.
- Stampini V, Bortoluzzi S, Allara E, Amadori R, Surico D, et al. (2019) The use of Complementary and Alternative Medicine (CAM) among Italian children: A cross-sectional survey. Complement Ther Med 47: 102184.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences