The Role of Thyroid Stimulating Hormone in Nephrolithiasis Associated with Chronic Kidney Disease
Sameena Iqbal1*, Sero Andonian1, Davine Yang2, Celena Scheede-Bergdahl2 and Khashayar Rafat Z1
1Department of Medicine, McGill University, CUISSS West Island, Pointe Claire, QC, Canada
2Department of Kinesiology, McGill University, CUISSS West Island, Pointe Claire, QC, Canada
- *Corresponding Author:
- Sameena Iqbal
Department of Medicine, McGill University
CUISSS West Island, Pointe Claire, QC
Canada
E-mail: sameena.iqbal@mcgill.ca
Received Date: October 01, 2021 Accepted Date: October 15, 2021 Published Date: October 22, 2021
Citation: Iqbal S, Andonian S, Yang D, Bergdahl SC, Rafat ZK, et al. (2021) The Role of Thyroid Stimulating Hormone in Nephrolithiasis Associated with Chronic Kidney Disease. J Nephrol Urol Vol.5 No.4:20.
Abstract
Introduction: The prevalence of nephrolithiasis in Chronic Kidney Disease (CKD) is 5%-10%. To better understand the relationship between thyroid function and nephrolithiasis in the CKD population, we conducted a retrospective study with the main objective to identify the prevalence of nephrolithiasis in CKD and explore the relationship between TSH hormone level and nephrolithiasis.
Methods: A retrospective cohort study was conducted in a community nephrology clinic in Quebec, Canada that included clinical and demographic data collection in an electronic format. The clinical information collected was from April 1, 2015 until December 30, 2019. The outcome of interest was the prevalence of nephrolithiasis, and the exposure variable of TSH level greater than 2.22 μIU/l was analysed by applying unconditional and adjusted generalized linear and logistic regression models.
Findings: The 310 charts were reviewed. The subjects had a median age of 73 years (IQR (interquartile range) 29-99), 58.3% was male, 12.8% had a diagnosis of hypothyroidism and a diagnosis of diabetes mellitus was made in 43.3%. The overall prevalence of nephrolithiasis 10.2% and was 9.4%, 14%, 6%, and 4.4% within the CKD groups combined, Grade 1 and 2,3,4 and 5 respectively. When certain generalized linear regression models were applied, an adjusted odds ratio of 2.38 (CI 95%: 1.08-5.27) was calculated for a TSH level>2.22 μIU/L (Q2), for the presence of nephrolithiasis on baseline CT scan of the abdomen.
Discussion: Our study shows a significant prevalence of nephrolithiasis in CKD, with a higher proportion of kidney stones in the early stages of the renal disease. TSH levels above 2 uIU/L have more than a two-fold higher risk of forming kidney stones. Further studies that address the target thyroxine level to resolve kidney stone formation will be important.
Keywords
Nephrolithiasis; Chronic kidney disease; Thyroid stimulating hormone
Introduction
Among Canadians with the Chronic Kidney Disease (CKD) population that make up 10%-12.5% of the population, the rates of asymptomatic nephrolithiasis are unknown [1]. In the general population, one study showed that 8.6% had asymptomatic kidney stones in the retrospective cohort of 1353 studies using radiological data [2]. In the community, the formation of nephrolithiasis is a result of urinary supersaturation of elements such as calcium, phosphate, oxalate, uric acid or cysteine [3]. Certain dietary components, such as reduced fluid and calcium intake, increased intake of carbohydrates, and excessive sodium and protein in the daily consumption, augment the risk of nephrolithiasis development and recurrence [4]. Higher body index and decreased physical activity are also been documented as risk factors for nephrolithiasis [3]. Hypothyroidism is related to insulin resistance, and to hyperuricemia [5]. The relationship of hypothyroidism to nephrolithiasis is not well described.
The aims of the present study were
• To determine the prevalence of asymptomatic nephrolithiasis in patients attending chronic kidney disease clinic.
• To compare thyroid stimulating hormone levels to presence of nephrolithiasis on radiological examination.
Materials and Methods
The study protocol was granted research ethics approval from the St. Mary’s Hospital Research Ethics Board, meeting the criteria for Helsinki declaration. We conducted a retrospective study utilizing a cohort of individuals followed at a nephrology clinic of a community hospital in Quebec. From over 1000 clinic charts, a random sample of 333 patients from the nephrology clinic of a community hospital was identified by the nephrology team and all data was collected and entered into an electronic system. Those subjects who met the inclusion criteria were 310. The subjects were entered in an electronic database which collected data from the following data sources laboratory data from Reflections database, clinical examination, medication list and demographical data from clinic charts, and radiological data from web-based PACs (Picture Archiving and Communication Systems) database. The period of collection was from April 1, 2015 until December 30, 2019.
The inclusion criteria were age ≥ 18 years, diagnosis of CKD as described with three consecutive eGFR readings of less than or equal to 90 ml/min/1.73 m2 and life expectancy of greater than 6 months. Six hundred and ten individuals were excluded if they were noted to have acute kidney injury, expected to require renal replacement therapy within 3 months, or moved to another health care facility. Cohort entry was defined as the date the individual met the diagnostic criteria of CKD.
The variables collected at cohort entry were as follows: age (at baseline assessment), gender (male or female), race (Caucasian, Arab, Asian, Black, Europe, South American), diabetes mellitus status (yes) cause of renal disease as documented in the chart, comorbidities (coronary artery disease, congestive heart failure, cerebrovascular disease, peripheral vascular disease, hypertension, pacemaker, chronic obstructive pulmonary disease, sleep apnea, cirrhosis, autoimmune disease, cancer history, deep venous thrombosis, dyslipidemia, dementia, hypothyroidism, gastroesophageal reflux, gout, and atrial fibrillation), height (m2, weight (kg), blood pressure (mmHg), baseline eGFR (ml/min/1.73 m2), baseline CKD grade, hemoglobin (mg/L), sodium (mmol/L), potassium (mmol/L), calcium (mmol/L), phosphate (mmol/L), TSH μIU/L, hemoglobin A1c (%), proteinuria (mg/L) and uric acid umol/L).
Sample size calculation
For calculation of sample size using logistic regression for presence of stone, the assumption of 5% proportion of individuals with TSH level above 2.22 IU/L compared to 10% of those with TSH level above 2.22 IU/L in the effect size of 0.5 and power of 90% and alpha error of 0.05, sample size required is 263.
Outcomes
The prevalence of asymptomatic nephrolithiasis by radiological report was calculated in the overall population and by CKD grades. The TSH levels were categorized by greater than 60th percentile (Q2) as well. Nephrolithiasis was defined as stone reported in the urinary tract on the first CT Scan of the abdomen after the first visit at the nephrologist office.
Statistical analyses
All prevalence data was estimated as a percentage with 95% confidence intervals using binomial proportions. Continuous variables were summarized as medians, ranges, means and standard deviations. The effect size and statistical significance for TSH level percentiles was explored for 10th, 25th, 33rd, 50th, 55th, 60th, 66th, 75th, 95th percentiles. Due to the largest effect size and statistical significance, the relationships between TSH level by 60th percentile and nephrolithiasis were utilized by applying unconditional and adjusted generalized linear regression and logistic regression models, respectively. Variables found statistically significant in the bivariate analyses were included in the final logistic regression models.
Results
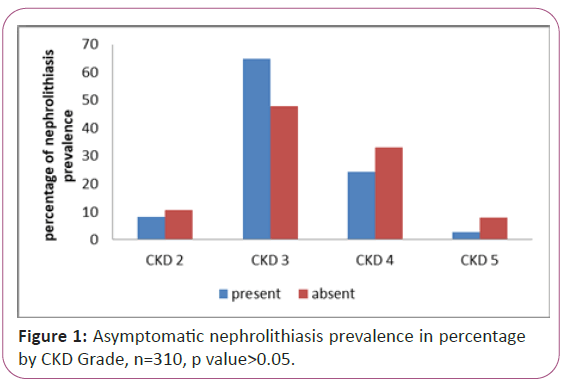
The 310 subjects had a median age of 73 (IQR 29-99) years, male gender 58.3% (182/312), 12.8% had a diagnosis of hypothyroidism (40/312) and a diagnosis of diabetes mellitus was made in 43.3% (135/312) (Table 1). Their baseline eGFR was 34 ml/min/1.73 m2 (IQR 9-93). The follow up period was 24.4 (IQR 0.93-103.5) months (Table 1). When the subjects were divided into chronic kidney disease categories (less than 15 ml/min/1.73 m2, 15-30 ml/ min/1.73 m2, 30-60 ml/min/1.73 m2 and >60 ml/min/1.73 m2) a progressive decrease in proportion of nephrolithiasis prevalence (Figure 1). The overall prevalence of nephrolithiasis 10.2% and was 9.4%, 14%, 6%, and 4.4% within the CKD groups combined, Grade 1 and 2, Grade 3, Grade 4 and Grade 5, respectively.
| Demographics | Number of subjects | Median/Proportion | IQR/ratio |
|---|---|---|---|
| Age | 310 | 73 | 29-99 |
| Gender: Male | 310 | 58.1% | 180 |
| height | 297 | 1.68 meters | 0.91-1.7 |
| weight | 299 | 78.4 kg | 34-150 |
| BMI | 294 | 27.8 | 16.8-48.8 |
| Race Caucasian other |
310 | 59.4% 40.6% |
184 126 |
| Comorbidities | |||
| Haemoglobin<100 g/l | 310 | 15.5% | 48 |
| Diabetes mellitus | 310 | 43.2% | 134 |
| Dementia | 310 | 2.3% | 7 |
| Pacemaker | 310 | 6.1% | 19 |
| Gout | 310 | 14.2% | 44 |
| GERD | 310 | 10% | 31 |
| Atrial fibrillation | 310 | 12.3% | 38 |
| Peripheral vascular disease | 310 | 11.3% | 35 |
| Coronary artery disease | 310 | 26% | 80 |
| Congestive heart failure | 310 | 13.5% | 42 |
| Cancer | 310 | 31% | 95 |
| Liver disease | 310 | 2.3% | 7 |
| COPD | 310 | 16.8% | 52 |
| Deep venous thrombosis | 310 | 4.2% | 13 |
| Dyslipidemia | 310 | 34.5% | 107 |
| Hematuria (microscopic) | 296 | 32.4% | 96 |
| Proteinuria | 296 | 45% | 148 |
| Hypothyroidism | 310 | 12.9% | 40 |
| History of kidney stone | 310 | 15.8% | 49 |
| Urological intervention | 310 | 0 | 0-9 |
| Clinic variable | |||
| Systolic blood pressure | 304 | 141 | 88-239 |
| Diastolic blood pressure | 304 | 75 | 40-104 |
| Heart rate | 305 | 71 | 49-123 |
| Medication | |||
| Levothyroxine | 39 | 88 | 0-225 |
| Laboratory | |||
| Thyroid stimulating hormone | 211 | 1.85 | 0.06-109.5 |
| Baseline eGFR ml/min/1.73 m2 | 312 | 34 | 9-93 |
| CKD grade 2 | 32 | 10.3% | |
| CKD grade 3 | 157 | 50.0% | |
| CKD grade 4 | 100 | 32.3% | |
| CKD grade 5 | 23 | 7.4% | |
| Serum creatinine | 312 | 147 | 63-626 |
| Parathyroid hormone | 227 | 9.1 | 1.4-107.4 |
| Vitamin D 25 OH | 175 | 83 | 5-362 |
| Vitamin D 1-25 OH | 120 | 90 | 18-278 |
| Haemoglobin | 305 | 123 | 76-172 |
| Serum sodium | 304 | 139 | 132-145 |
| Serum potassium | 304 | 4.5 | 2.6-6.2 |
| Serum bicarbonate | 263 | 26 | 14-33 |
| Blood urea | 278 | 10.4 | 2.9-42.8 |
| Serum albumin | 293 | 39 | 19.5-47 |
| Serum uric acid | 287 | 393 | 117-879 |
| Total cholesterol | 267 | 4.3 | 1.92-8.63 |
| HDL | 262 | 1.14 | 0.54-2.64 |
| LDL | 262 | 2.3 | 0.71-5.85 |
| Serum calcium | 216 | 2.38 | 1.19-2.76 |
| Ionized calcium | 78 | 1.3 | 1.13-1.39 |
| Serum phosphate | 290 | 1.2 | 0.63-2.26 |
| C reactive protein | 239 | 5.5 | 2.03-293 |
| Ferritin | 277 | 72 | 2.22-1022 |
| Hb A1c | 273 | 5.8 | 4.8-11.3 |
| Urine albumin/creatinine | 269 | 10.2 | 0.17-1414 |
Table 1: Overall, patient demographics, comorbidities, clinic visit, laboratory, and radiological data for study population.
Figure 1: Asymptomatic nephrolithiasis prevalence in percentage by CKD Grade, n=310, p value>0.05.
On the bivariate analysis, there was a tendency toward statistical difference in both decreased LDL cholesterol, presence of haematuria and higher serum phosphate level and parathyroid hormone level of greater than 17.1 pmol/l that was associated with nephrolithiasis presence on the report (Table 2). There was statistically significant difference on the serum phosphate and urine albumin/creatinine ratio on the identification of nephrolithiasis on the radiological results (Table 2).
| Nephrolithiasis | Absence of nephrolithiasis | P value | ||||
|---|---|---|---|---|---|---|
| Demographics | Number of subjects | Median/ Proportion |
IQR/ratio | Median/ proportion |
IQR/ratio | |
| Age (years) | 310 | 74 | 42-88 | 73 | 29-99 | 0.7671 |
| Gender: Male | 310 | 73% | 27/37 | 56% | 153/280 | 0.0502 |
| Height (meters) | 297 | 1.68 | 1.52-1.85 | 1.67 | 0.91-1.7 | 0.5829 |
| Weight | 299 | 75 | 50.9-108.4 | 78.7 | 34.02-150 | 0.6987 |
| BMI | 294 | 28.3 | 20.3-38.7 | 27.8 | 16.8-48.8 | 0.7369 |
| Race Caucasian other |
310 | 67.6 32.4 |
25/37 | 58.2 41.8 |
159/276 | 0.4002 |
| Comorbidities | ||||||
| Hemoglobin<100 g/l | 310 | 8.11 | 3/37 | 16.5 | 45/273 | 0.2317 |
| Diabetes mellitus | 310 | 46 | 17/37 | 42.9 | 117/273 | 0.7219 |
| Dementia | 310 | 0 | 2.6% | 7/273 | 1.0000 | |
| Pacemaker | 310 | 2.7% | 1/37 | 6.6 | 18/273 | 0.3545 |
| Gout | 310 | 18.9% | 5/37 | 13.6% | 40/273 | 0.3801 |
| GERD | 310 | 8.1% | 3/37 | 10.3 | 28/273 | 1.0000 |
| Atrial fibrillation | 310 | 10.8 | 4/37 | 12.5 | 34/273 | 0.7748 |
| Peripheral Vascular disease | 310 | 5.4 | 2/37 | 12.1 | 33/273 | 0.4026 |
| Coronary artery disease | 310 | 21.6% | 8/37 | 26.4% | 72/273 | 0.5353 |
| Congestive heart failure | 310 | 5.6% | 2/37 | 14.5% | 40/276 | 0.1395 |
| Cancer | 310 | 34% | 12/37 | 30.4% | 83/273 | 0.8016 |
| Liver disease | 310 | 0 | 2.7% | 7/273 | 1.0000 | |
| COPD | 310 | 16.2% | 6/37 | 16.9 | 46/273 | 0.9229 |
| Deep venous thrombosis | 310 | 8.3 | 3/37 | 3.7 | 10/273 | 0.1930 |
| Dyslipidaemia | 310 | 48.7% | 18/37 | 32.6 | 89/273 | 0.0540 |
| Haematuria | 310 | 32.4% | 15/37 | 30.5 | 79/182 | 0.1529 |
| Proteinuria | 310 | 40.5% | 15/37 | 46 | 119/273 | 0.0819 |
| Hypothyroidism | 310 | 10.8% | 3/37 | 13.21% | 37/273 | 0.6858 |
| History of Stone^ | 310 | 100% | 37/37 | 4.4% | 12/273 | <0.0001 |
| Urological intervention* | 310 | 0 | 0-9 | 0 | 0 | <0.0001 |
| Clinic variable | ||||||
| Systolic blood pressure | 306 | 140 | 92-191 | 141 | 88-239 | 0.8928 |
| Diastolic blood pressure | 306 | 74 | 54-100 | 75 | 40-104 | 0.8865 |
| Heart rate | 307 | 72 | 52-102 | 71 | 49-123 | 0.6587 |
| Medication | ||||||
| Levothyroxine | 39 | 50 | 50-112 | 50 | 0-225 | 0.3603 |
| Laboratory | ||||||
| Thyroid stimulating hormone | 211 | 2.3 | 0.65-6.56 | 1.82 | 0.06-109.5 | 0.3768 |
| TSH hormone greater than 2.22 IU/L* | 310 | 40.5% | 15/36 | 24.9% | 68/276 | 0.0439 |
| Baseline eGFR ml/min/1.73 m2 | 310 | 37 | 13-86 | 34 | 9-93 | 0.1317 |
| Serum creatinine | 310 | 139 | 81-319 | 148 | 63-626 | 0.2517 |
| Parathyroid hormone* | 227 | 5.6 | 1.4-37.7 | 9.4 | 1.4-107.4 | 0.0026 |
| Vitamin D 25 OH | 175 | 77 | 27-149 | 83.5 | 5-362 | 0.4843 |
| Vitamin D 1-25 OH | 120 | 92 | 39-278 | 90 | 18-254 | 0.6842 |
| Haemoglobin | 307 | 123 | 96-160 | 123 | 76-172 | 0.3918 |
| Serum sodium | 306 | 139 | 132-148 | 139 | 130-145 | 0.9880 |
| Serum potassium | 306 | 4.4 | 3.8-5.8 | 4.5 | 2.6-6.2 | 0.2179 |
| Serum bicarbonate | 265 | 26 | 20-30 | 26 | 14-33 | 0.4173 |
| Blood urea | 280 | 9.2 | 4.6-26 | 10.7 | 2.9-42.8 | 0.0623 |
| Serum albumin | 295 | 39 | 28-44 | 39 | 19.5-47 | 0.4783 |
| Serum uric acid | 289 | 373 | 172-604 | 397 | 117-879 | 0.0779 |
| Total cholesterol* | 269 | 4.0 | 2.35-6.24 | 4.4 | 1.92-8.63 | 0.0261 |
| HDL | 264 | 1.11 | 0.6-1.98 | 1.15 | 0.54-2.64 | 0.1040 |
| LDL* | 264 | 2.06 | 0.56-3.48 | 2.36 | 0.72-5.85 | 0.0136 |
| Serum calcium | 218 | 2.38 | 2.1-2.63 | 2.38 | 1.19-2.76 | 0.9800 |
| Ionized calcium | 78 | 1.25 | 1.21-1.29 | 1.27 | 1.13-1.39 | 0.4353 |
| Serum phosphate* | 291 | 1.105 | 0.69-1.59 | 1.23 | 0.63-2.26 | 0.0004 |
| C reactive protein | 241 | 4 | 4-72.2 | 5.6 | 2.03-293 | 0.2614 |
| Ferritin | 279 | 61 | 9-486 | 73 | 2.22-1022 | 0.5127 |
| HbA1c | 275 | 5.8 | 4.4-9.6 | 5.8 | 4.8-11.3 | 0.7584 |
| Urine albumin/creatinine | 270 | 61 | 0.41-681 | 12.5 | 0.17-1414 | 0.1453 |
Note:*p value<0.05
Table 2: Patient demographics, comorbidities, clinic visit, laboratory and radiological data for study population categorized by nephrolithiasis status n=310.
The 60th percentile for TSH level was 2.22 uIU/L. TSH greater than 2.22 uIU/l had an unadjusted odds ratio of 2.06 (CI 95%: 1.01-4.19) and an adjusted odds ratio of 2.38 (CI 95%: 1.08-5.27) for nephrolithiasis on the CT scan of the abdomen or ultrasound at baseline assessment (Table 3).
| Variable | Unadjusted odds ratio (95% CI) | Adjusted odds ratio (95% CI ) | P value |
|---|---|---|---|
| TSH>2.22 uIU/l | 2.06 (1.01-4.19) | 2.38 (1.08-5.27) | 0.0324 |
| TSH ≤ 2.22 uIU/l (reference) | 1.0 | ||
| Age (years) | 1.01 (0.98-1.04) | 1.01 (0.98-1.04) | 0.5807 |
| Parathyroid hormone>17.1 pmol/l | 0.71 (0.24-2.12) | 0.94 (0.27-3.26) | 0.9194 |
| eGFR at baseline | 1.014 (0.994-1.034) | 1.004 (0.98-1.03) | 0.7564 |
| Serum phosphate | 0.04 (0.01-0.29) | 0.04 (0.004-0.35) | 0.0040 |
| Serum uric acid | 0.996 (0.993-1.000) | 1.00 (0.99-1.00) | 0.0706 |
| Gender female | 0.47 (0.22-1.01) | 0.69 (0.29-1.62) | 0.3958 |
Table 3: Unadjusted and adjusted logistic regression models for TSH level greater than 2.22 IU/L and nephrolithiasis N=277.
The multivariate logistic regression model was adjusted for baseline eGFR, age, serum phosphate, parathyroid hormone above 17.1 pmol/l and serum uric acid (Table 3).
Discussion
Interestingly, the prevalence of asymptomatic nephrolithiasis in CKD is similar to the symptomatic nephrolithiasis in the general population. As the CKD Grade at clinic presentation increased in severity, the prevalence of asymptomatic stone disease decreased. One plausible explanation is decreased eGFR to leads less filtration of elements such as calcium, phosphate, uric acid, and oxalate that result in decrease supersaturation of urine. Another is healthier individuals eat and maintain muscle mass, whereas those who develop malnutrition have decreased appetite and poor intake of the above stone forming elements. It is well-recognized in the literature that nephrolithiasis results in kidney scarring and decreased renal function [6].
Hypothyroidism is associated with hyperparathyroidism, and radiation exposure is considered as one possible cause [7]. Another possibility is the low vitamin D 25 hydroxyl levels that have been reported in subclinical hypothyroidism that can facilitate the development of secondary hyperparathyroidism and hypercalcaemia [8]. Vitamin D supplementation improves the TSH levels. TSH levels are increased in individuals with insulin resistance, the mechanism is unclear. TSH has been shown to stimulate the Glucose transporter 2 of ß2 cells of pancreas that further promotes the secretion of insulin [9]. Hyperinsulinemia is also associated with clinically significant hypercalcaemia with a theory of diminished resorption of calcium from the proximal renal tubule, postprandial. With the relationship of obesity and diabetes, hypothyroidism is associated with hyperuricemia and uric acid stones. Decreased renal perfusion resulting in decreased glomerular filtration rate seen in hypothyroidism is postulated to be due to the thyroxine deficient state resulting in a bradycardic effect on the sinus node and ultimately lowering the cardiac output. Another potential mechanism for TSH to affect stone formation is the effect it may have to the calcium sensitive receptors on the ureters that control ureteric peristalsis [10].
Conclusion
The limitations of the study include the method of diagnosis was radiologist dependent with one reader observation. The retrospective nature of the study in a specialized nephrology clinic for CKD will represent a higher proportion of nephrolithiasis. The small sample size and cross-sectional design only allows identification of an association between TSH levels and nephrolithiasis not a definite causal relationship.
Further studies are required to re-evaluate the target TSH level and treatment goals for hypothyroidism in renal disease.
Disclosures
None
Funding
None
Acknowledgments
None
References
- Arora P, Vasa P, Brenner D, Iglar K, McFarlane P, et al. (2013) Prevalence estimates of chronic kidney disease in Canada: results of a nationally representative survey. Can Med Assoc J 185: E417-E423
- Bansal DA, Hui J, Goldfarb SD (2009) Asymptomatic nephrolithiasis detected by ultrasound. Clin J Am Soc Nephrol 4: 680-684
- Bao Y, Tu X, Wei Q (2020) Water for preventing urinary stones. Cochrane Database Syst Rev. 11: CD004292
- Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ, et al. (1997) Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med 126: 497-504
- Aune D, Mahamat-Saleh Y, Norat T, Riboli E (2018) Body fatness, diabetes, physical activity and risk of kidney stones: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol 11: 1033-1047
- D'Costa M, Savcic-Kos R, Huang J, Rule AD, Murali N, et al. (2016) Urological procedures in urolithiasis and their association with chronic kidney disease. Clin Med Res 14: 75-82
- Ahi S, Dehdar MR, Hatami N (2020) Vitamin D deficiency in non-autoimmune hypothyroidism: A case-control study. BMC Endocr Disord. 20: 41
- Talaei A, Ghorbani F, Asemi Z (2018) The Effects of vitamin D supplementation on thyroid function in hypothyroid patients: A randomized, double-blind, placebo-controlled trial. Indian J Endocrinol Metab 22: 584-588
- Cortizo AM, Chazenbalk GD, Gagliardino de EE, Garcia ME, Pisarev MA, et al. (1987) Thyroid hormone binding and deiodination by pancreatic islets: relationship with the in vitro effect upon insulin secretion. Acta Endocrinol 116: 66-72
- Burdyga T, Lang RJ (2019) Excitation-contraction coupling in ureteric smooth muscle: mechanisms driving ureteric peristalsis. Adv Exp Med Biol 1124: 103-119
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences