ISSN : 2249 - 7412
Asian Journal of Plant Science & Research
The Interaction between Plant and Endophytes for Conferring Tolarence to Biotic and Abiotic Stress in Presence of Acc (1-Aminocyclopropane-1-Carboxylate) Deaminase: A Stress Weapon
Shivangi H Zaveri* and Sumita Dasgupta
Department of Biotechnology, Bhagwan Mahavir College of Basic and Applied Sciences, Surat, India.
- *Corresponding Author:
- Shivangi H Zaveri
- Department of Biotechnology,
Bhagwan Mahavir College of Basic and Applied Sciences, Surat,
India,
E-mail: zaverishivangi@gmail.com
Received date: August 01, 2022, Manuscript No. AJPSKY-22-14147; Editor Assigned date: August 03, 2022, PreQC No. AJPSKY-22-14147(PQ); Reviewed date: August 15, 2022, QC No. AJPSKY-22-14147; Revised date: August 24, 2022, Manuscript No. AJPSKY-22-14147(R); Published date: August 31, 2022, DOI: 10.36648/2249- 7412.12.8.307
Citation: Zaveri SH, Dasgupta S (2022) The Interaction between Plant and Endophytes for Conferring Tolarenc-e to Biotic and Abiotic Stress in Presence of Acc (1-Aminocyclopropane-1-Carboxylate) Deaminase: A Stress Weapon. Asian J Plant Sci Res Vol.12 No.8:307.
Abstract
Plants are exposed to various biotic and abiotic stresses, there by growth become arrested due to accumulation of stress ethylene. Drought conditions may persist for several months to years and can significantly affect plant health and survival. In this regard, various technologies including traditional breeding and genetic engineering are used to cope with drought stress. Some Plant Growth-Promoting Rhizobacteria (PGPRs) has the capacity to stimu- late plant growth even under stressed conditions by reducing ethylene levels in plants. PGPR contain the enzyme 1-Aminocyclopropane-1-Carboxylate (ACC) deaminase that assists plant growth and development by minimization of plant ethylene levels. ACC deaminase has the capacity to metabolize the ACC into ketobutyrate and ammonia and thereby checks the production of ethylene. Indirectly, endophytic bacteria also improve osmotic adjustment, relative water content and antioxidant activity of inoculated plants. Altogether, these bacterial-mediated drought tolerance and plant growth–promoting processes continue even under severe drought conditions which lead to enhanced plant growth promotion and yield. In this review we are trying to insight a brief outline of bacterial ACC deaminase and their role in alleviation of water stress tolerance in plants.
Keywords
1-Aminocyclopropane-1-Carboxylate (ACC); ACC deaminase; PGPR; Ethylene; Biotic and abiotic stress.
Introduction
Plant growth involves the synergistic activity of many different life forms in a highly complex environment. While it is still a common perception that interactions between plants and microbes often lead to plant diseases, pathogens represent only a small fraction of the microbial communities associated with plants. A field of growing plants is a complex of microbial activity, comprising soil microbes, atmospheric microbes, plant surface microbes and internal, plant-colonizing microbes. Bacterial and fungal endophytes occur within plant organs and tissues causing no ill ef- fects and some actually benefit plant health. The array of endophytes is large and variable in many plants and their exact composition depends upon many factors. Selection of cultivars of a desired plant that are capable of developing positive interactions with endophytes may actually boost plant productivity and enhance soil health, as many of these microbes also establish large populations in the rhizosphere [1,2].
Plant–Endophyte interaction
Successful endophyte-plant interactions require colonization of a plant by the endophytes. Endophytic fungi reside entirely within the plant, such as the root, stem and leaves. Bacterial endophytes also primarily occur intercellular and can be found completely belowground, aboveground, or both. Following root introduction, stem colonization oc- curred, followed by leaf colonization via the transpiration stream. Other reports have also suggested that colonization of the plant can occur via intercellular space movement. This colonization of intercellular spaces by both fungal and bacterial endophytes is not surprising, as these spaces are mineral rich environments, containing potassium, calcium, sulfur, phosphorous and chlorine, as well as numerous sugars and non-carbohydrate metabolites, including various amino acids and organic acids [2,3]. Endophyte alteration of apoplastic pH can modify enzyme activities and sugar uptake of host cells, as well as inorganic sugar concentrations for the colonizing microbes. Hence, this environment is supportive of endophyte growth, allowing cycling of compounds between the endophyte and the plant.
Ethylene and its biosynthesis in higher plants
Ethylene hormones are responsible for various plant growth parameters, fruit ripening, senescence and abscission [4]. Various environmental stresses including drought stress lead to the enhanced ethylene production and might reach to the inhibitory levels of plant growth. To reduce ethylene levels in plants, bacteria producing ACC deaminase repre- sents as a key physiological strategy to continue growth under stressed conditions. Chemical inhibitors of ethylene synthesis or action completely block ripening in fruits and senescence in flowers of many plant species. Bacteria which produce ACC deaminase cleaves the immediate precursor of ethylene, i.e., ACC in plants, producing ammonia and α-ketobutyrate that lead to reduced ethylene level in the plants and enhance growth. The Pseudomonas sp. ACP reported first bacterium to synthesize ACC deaminase. Lately, numerous bacteria including endophytic bacteria have been found to synthesize ACC deaminase [5].
Plant stress and ethylene production
When plants are exposed to conditions that threaten their ability to survive, the same mechanism that produces ethyl- ene for normal development instead produces “stress ethylene” which may be defined as an acceleration of ethylene biosynthesis associated with biological and environmental stresses and pathogen attack [6].
The ACC deaminase-producing bacteria play a key role in plant growth and development through management of ethylene biosynthesis in plant tissues experiencing by a number of biotic and abiotic factors. Ethylene regulates many plant developmental processes such as germination, root and shoot elongation, abscission, senescence, flowering and fruit ripening and the responses to environmental stress. One of the most impacting effects of ethylene on plant growth occurs as a result of stress conditions. In this instance, the stressed plant first produces a small peak of ethyl- ene that activates the transcription of various plant defensive genes [7]. Subsequently, the stressed plant synthesizes a high level of ethylene (termed stress ethylene) that ultimately can lead to plant premature senescence and death. In fact, some of the effects of stress cannot solely be attributed to the stress itself but are also due to autocatalytic ethylene synthesis [8].
Mechanism of ACC deaminase action
ACC deaminase is a multimeric enzyme (homodimeric or homotrimeric) with a subunit molecular mass of approxi- mately 35-42 kDa. It is a sulfhydral enzyme in which one molecule of the essential cofactor of Pyridoxal 5-Phosphate (PLP), is tightly bound to each subunit. It falls under the group of those enzymes which requires the co-factor pyri- doxal 5’-phosphate for enzymatic activity. Interestingly, this enzyme is localized in cytoplasm so that the substrate ACC must be exuded by plant tissues and subsequently taken up by an ACC deaminase-containing microorganism before it is cleaved [6,8]. The enzyme ACC deaminase (EC: 4.1.99.4) which catalyzes the cleavage of ACC to am- monia and α-ketobutyrate was first discovered in 1978. X-ray crystallographic analysis reveals that ACC deaminase folds into two domains, each of which has an open twisted α/β structure that is similar to the subunit of the enzyme tryptophan synthase. Thus ACC deaminase fits into the tryptophan synthase family [9].
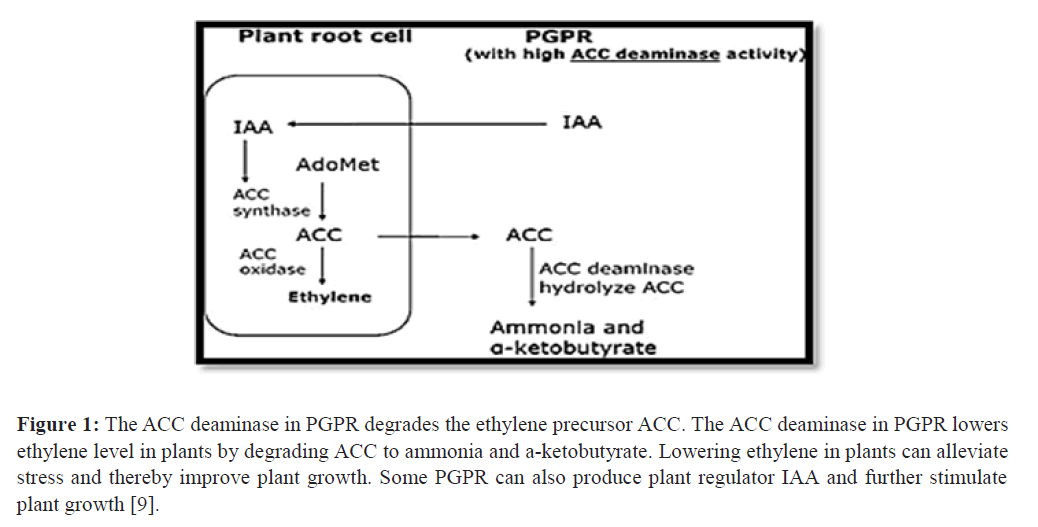
Ethylene is synthesized from S-adenosyl L-methionine (AdoMet) by way of the intermediate ACC. Ethylene biosyn- thesis consists of three steps;
1. L-methionine is converted to AdoMet, a reaction catalyzed by methionine S-adenosyl transferase. AdoMet is also utilized in other cellular reactions such as ethylation and polyamine synthesis.
2. The conversion of AdoMet to ACC which is catalyzed by ACC synthase. The ACC synthase step is considered to be the rate-limiting step in the pathway.
3. ACC is further metabolized to ethylene, carbon dioxide and cyanide by ACC oxidase.
The limiting step in plant ethylene biosynthesis is generally considered to be the conversion of SAM to ACC, indicat- ing the key role of ACC in plant ethylene production Figure 1.
Figure 1: The ACC deaminase in PGPR degrades the ethylene precursor ACC. The ACC deaminase in PGPR lowers ethylene level in plants by degrading ACC to ammonia and a-ketobutyrate. Lowering ethylene in plants can alleviate stress and thereby improve plant growth. Some PGPR can also produce plant regulator IAA and further stimulate plant growth [9].
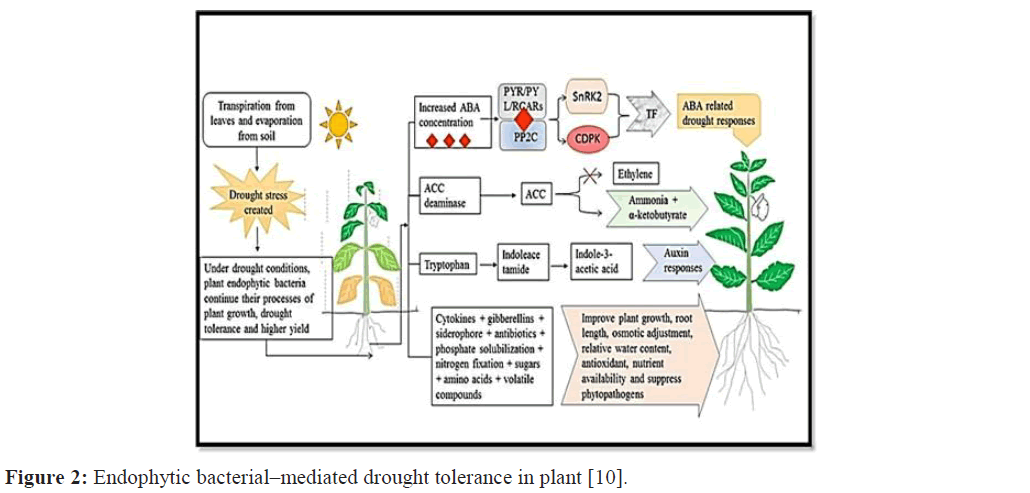
Numerous rhizospheric bacteria producing ACC deaminase have been found to enhance drought tolerance. However, limited evidences have been reported about the production of ACC deaminase by endophytic bacteria. In regard to the role of ACC deaminase–producing endophytic bacteria in drought stress, studied that ACC deaminase–producing endophytic bacteria, burkholderia phytofirmans, enhanced drought tolerance in maize [10]. In addition, burkholderia phytofirmans also enhanced drought tolerance in panicum virgatum Figure 2.
Figure 2: Endophytic bacterial–mediated drought tolerance in plant [10].
There are several reports of transformed bacterial strains receiving the ACC deaminase gene (acdS gene). Further- more the presence of acdS gene was reported in variety of microorganisms including, azospirillum, rhizobium, agro- bacterium, achromobacter, burkholderia, ralstonia, pseudomonas and enterobacter. The gene acdS is found to be widely distributed in different pseudomonas strains [10].
A model was proposed by which plant growth-promoting bacteria can lower plant ethylene levels and in turn facili- tates plant growth [11]. In this model the plant growth-promoting bacteria bind to the surface of a plant. In response to tryptophan and other small molecules in the plant exudates, the bacteria synthesize and secrete Indole-3-Acetic Acid (IAA), some of which is taken up by the plant. This IAA together with endogenous plant IAA can stimulate plant cell proliferation, plant cell elongation or induce the transcription of ACC synthase which is the enzyme that catalyzes the formation of ACC. Some of the ACC is exuded from seeds, roots or leaves along with other small molecules nor- mally present in these exudates and may be taken up by the bacteria and subsequently cleaved by the enzyme, ACC deaminase, to ammonia and α-ketobutyrate. In this model, the bacterium acts as a sink for plant ACC and as a result of lowering either the endogenous or the IAA-stimulated ACC level, the amount of ethylene in the plant is also reduced. As a direct consequence of lowering plant ethylene levels, plant growth-promoting bacteria that possess the enzyme ACC deaminase can reduce the extent of ethylene inhibition of plant growth following a wide range of stresses. Thus, plants grown in association with these bacteria should have longer roots and shoots and be more resistant to growth inhibition by a variety of ethylene-inducing stresses.
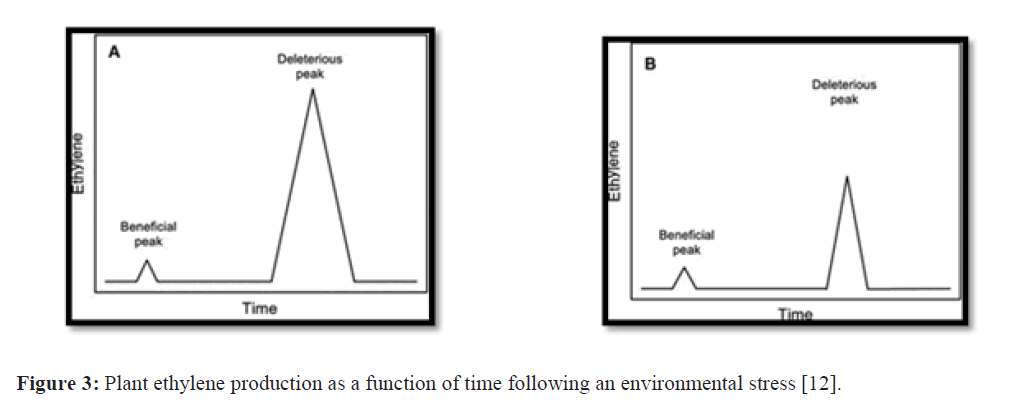
The question arises, as to how bacterial ACC deaminase can selectively lower deleterious ethylene levels but not af- fect the small peak of ethylene that is thought to activate some plant defense responses (Fig. 2A). As discussed later in this review, ACC deaminase is generally present in bacteria at a low level until it is induced and the induction of enzyme activity is a relatively slow and complex process. Immediately following an environmental stress, the pool of ACC in the plant is low as is the level of ACC deaminase in the associated bacterium. Following the relatively rapid induction of a low level of ACC oxidase in the plant, it is likely that there is increased flux through this enzyme resulting in the first small peak of ethylene which is of sufficient magnitude to induce a protective/defensive response in the plant (Fig. 2B). With time, bacterial ACC deaminase is induced (by the increasing amounts of ACC that ensue from the induction of ACC synthase in the plant) so that the magnitude of the second, deleterious, ethylene peak is decreased significantly (Fig. 2B). The second ethylene peak may be reduced dramatically, but it is never completely abolished since ACC oxidase has a much higher affinity for ACC than does ACC deaminase [11,12]. Thus, when ACC deaminase-producing bacteria are present, ethylene levels are ultimately dependent upon the ratio of ACC oxidase to ACC deaminase Figure 3.
Figure 3: Plant ethylene production as a function of time following an environmental stress [12].
[(A) In the absence of any exogenous bacteria. (B) In the presence of an ACC deaminase producing plant growth- promoting bacterium. In both cases, there is an initial small peak of ethylene that is thought to activate transcription of plant defense genes, which is often difficult to detect, followed some time later by a much larger ethylene peak that can cause adverse responses in the plant. The amount of ethylene produced in response to an environmental stress is related to the plant age as well as the nature and severity of the stress.]
In normal condition, ACC deaminase present in bacteria at a low level. Similarly, in plant immediately following an environmental stress, the ACC level is low. However, rapid induction of a low level of ACC oxidase in the plant stim- ulates very low level of ethylene production, being sufficient to induce a protective/defensive response in the plant. Again due to action of ACC synthase, amounts of ACC increase in plants. With due course of time, bacterial ACC deaminase is also induced which regulates the deleterious ethylene action. Since ACC oxidase has a much higher af- finity for ACC than does ACC deaminase, therefore, when ACC deaminase-producing bacteria are present, ethylene levels are ultimately dependent upon the ratio of ACC oxidase to ACC deaminase [13].
Alternate chemical inhibitors of ethylene synthesis and bacterial ACC deaminse
Several different chemicals such as Amino-ethoxy-Vinyl-Glycine (AVG), Aminooxy-Acetic acid (AOA) and 1-Meth- ylcyclo-Propene (1-MCP) have been successfully used to lower the ethylene level [14]. In most cases these chemicals are expensive, less feasible or potentially harmful to the environment. On the other hand, use of PGPR containing ACC deaminase activity is more economical, environmental friendly and feasible in a natural soil and plant system. Moreover, the use of PGPR containing ACC deaminase activity is advantageous because ACC deaminase trait is com- mon among a number of PGPR species, which are native to the rhizosphere and consequently possess a vast array of survival potential in the rhizosphere and rhizoplane. In addition, PGPR possess several other traits like synthesis of auxins, gibberellins, cytokines and/or polyamines, which directly promote plant growth [15-17]. These characteristics make the selection of PGPR containing ACC deaminase more reliable than any other alternative.
In a number of studies, inoculation with PGPR containing ACC deaminase has been unequivocally shown to alter the endogenous levels of ethylene, which subsequently leads to changes in plant growth [18,19]. Some examples of plants inoculated with ACC deaminase containing bacteria and the physiological eVects of the latter have been described in Table 1.
| Plant Species | PGPR | Role | Reference |
|---|---|---|---|
| Brassica campestris | Methylobacterium fujisawaense | Bacterium promoted root elongation in canola. | 18 |
| Brassica napus | Alcaligenes sp., Bacillus pumilus, Pseudomonas sp., Variovorax paradoxus. | Inoculated plant demonstrated more vigorous growth than the control | 18 |
| Dianthus caryophyllus L. | Azospirillum brasilense Cd1843 | Inoculated cuttings produced longest roots. | 19 |
| Glycine max | Pseudomonas cepacia | Rhizobacterium caused an early soybean growth. | 20 |
| Pisum sativum L. | Rhizobium leguminosarum bv. viciae 128C53K | Bacterium enhanced nodulation in plants. | 21 |
| Vigna radiata L. | Pseudomonas putida | The ethylene production was inhibited in inoculated cuttings. | 22 |
| Zea mays L. | Enterobacter sakazakii 8MR5 Pseudomonas sp. 4MKS8 | Inoculation increased agronomic parameters of maize. | 22 |
Table 1: ACC deaminase containing bacteria and the physiological eVects.
Future and Conclusion
Plant rhizosphere engineering with ACC deaminase-producing bacteria can reduce the inhibition-effects of ethyl- ene and thereby increasing the plant growth. However, the application of PGPR containing ACC deaminase is very crucial agent to regulate the plant ethylene, thus helps the plant to live under adverse climatic condition [20]. On a large scale, application of PGPR strains having ACC deaminase activity might prove beneficial and could be a sound step towards sustainable crop production and conservation. It has been proved scientifically that the plant growth- promoting bacteria appear, in many cases, to present a superior alternative to the use of transgenic plants in different stress conditions [21]. Bacterial ACC-deaminase could have a definite benefit for field crops where the environment and degree of stresses such as salinity, drought, flooding etc. are not always predictable. Moreover, these PGPR strains can be used for the preparation of bio fertilizers [22]. For this purpose, it is mandatory to know how we can increase the stability of these PGPR strains under natural soil environment and to select the most efficient strains. Furthermore, future research should focus on.
i) How plant growth is altered under stress conditions
ii) Studying the interaction between PGPR strains and climatic factors under natural conditions and their influence on microbial growth
iii) Determining the survival of PGPR con- taining ACC-deaminase under diverse field conditions and
iv) Preparation of effective biofertilizers.
References
- Nadeem SM, Zahir ZA, Naveed M, Ashraf M (2010) Microbial ACC-deaminase: Prospects and applications for inducing salt tolerance in plants. Critical Reviews in Plant Sciences 29: 360-393.
[Crossref], [Google Scholar]
- Miliute I, Buzaite O, Baniulis D, Stanys V (2015) Bacterial endophytes in agricultural crops and their role in stress tolerance: A review. Zemdirbyste-Agriculture 102: 465-478.
[Crossref], [Google Scholar]
- Omomowo OI, Babalola OO (2019) Bacterial and fungal endophytes: Tiny giants with immense beneficial potential for plant growth and sustainable agricultural productivity. Microorganisms 7: 481.
[Crossref], [Google Scholar]
- Mei C, Flinn BS (2020) The use of beneficial microbial endophytes for plant biomass and stress tolerance improvement. Recent Pat Biotechnol 1: 81-95.
[Crossref], [Google Scholar]
- Trivedi G, Shah R, Patel P, Saraf M (2017) Role of endophytes in agricultural crops under drought stress: Current and future prospects. JAM 3: 174-188.
[Crossref], [Google Scholar]
- Kushwaha P, Kashyap PL, Bhardwaj AK, Kuppusamy P, Srivastava AK, et al. (2020) Bacterial endophyte mediated plant tolerance to salinity: Growth responses and mechanisms of action. World Journal of Microbiology and Biotechnology 36: 1-16.
[Crossref], [Google Scholar]
- Majeed A, Muhammad Z, Ahmad H (2018) Plant growth promoting bacteria: Role in soil improvement, abiotic and biotic stress management of crops. Plant Cell Reports 37: 1599-1609.
[Crossref], [Google Scholar]
- Ullah A, Nisar M, Ali H, Hazrat A, Hayat K, et al. (2019) Drought tolerance improvement in plants: An endophytic bacterial approach. Appl Microbiol Biotechnol 103: 7385-7397.
[Crossref], [Google Scholar]
- Husen E, Wahyudi AT, Suwanto A, Saraswati R (2018) Prospective use of 1-aminocyclopropane-1-carboxylate deaminase-producing bacteria for plant growth promotion and defense against biotic and abiotic stresses in peat-soil-agriculture. Microbiology Indonesia 2: 2.
[Crossref], [Google Scholar]
- Saleem M, Arshad M, Hussain S, Bhatti AS (2017) Perspective of Plant Growth Promoting Rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. Journal of Industrial Microbiology and Biotechnology 34: 635-648.
[Crossref], [Google Scholar]
- Gontia-Mishra I, Sasidharan S, Tiwari S (2014) Recent developments in use of 1-Aminocyclopropane-1-Carboxylate (ACC) deaminase for conferring tolerance to biotic and abiotic stress. Biotechnology letters 36: 889-898.
[Crossref], [Google Scholar]
- Glick BR, Cheng Z, Czarny J, Duan J (2007) Promotion of plant growth by ACC deaminase-producing soil bacteria. European Journal of Plant Pathology 119: 329-339.
[Crossref], [Google Scholar]
- Saraf M, Jha CK, Patel D (2010) The role of ACC deaminase producing PGPR in sustainable agriculture. In Plant Growth and Health Promoting Bacteria 18: 365-385. Springer, Berlin, Heidelberg.
[Crossref], [Google Scholar]
- Bal HB, Nayak L, Das S, Adhya TK (2018) Isolation of ACC deaminase producing PGPR from rice rhizosphere and evaluating their plant growth promoting activity under salt stress. Plant and Soil 366: 93-105.
[Crossref], [Google Scholar]
- Danish S, Zafar-ul-Hye M, Mohsin F, Hussain M (2020) ACC-deaminase producing plant growth promoting rhizobacteria and biochar mitigate adverse effects of drought stress on maize growth. PLoS One 15: e0230615.
[Crossref], [Google Scholar]
- Danish S, Kiran S, Fahad S, Ahmad N, Ali MA, et al. (2019) Alleviation of chromium toxicity in maize by fe fortification and chromium tolerant ACC deaminase producing plant growth promoting rhizobacteria. Ecotoxicology and Environmental Safety 185: 109706.
[Crossref], [Google Scholar]
- Zafar-ul-Hye M, Danish S, Abbas M, Ahmad M, Munir TM (2019) ACC deaminase producing PGPR Bacillus amyloliquefaciens and Agrobacterium fabrum along with biochar improve wheat productivity under drought stress. Agronomy 9: 343.
[Crossref], [Google Scholar]
- Gontia-Mishra I, Sapre S, Kachare S, Tiwari S (2017) Molecular diversity of 1-Aminocyclopropane-1-Carboxylate (ACC) deaminase producing PGPR from wheat (Triticum aestivum L.) rhizosphere. Plant and soil, 414: 213-227.
[Crossref], [Google Scholar]
- Moon YS, Ali S (2022) Possible mechanisms for the equilibrium of ACC and role of ACC deaminase-producing bacteria. Appl Microbiol Biotechnol 106: 877-887.
[Crossref], [Google Scholar]
- Chandra D, Srivastava R, Gupta VV, Franco CM, Sharma AK (2019) Evaluation of ACC-deaminase-producing rhizobacteria to alleviate water-stress impacts in wheat (Triticum aestivum L.) plants. Can J Microbiol 65: 387-403.
[Crossref], [Google Scholar]
- Siddikee MA, Chauhan PS, Anandham R, Han GH, Sa TM (2010) Isolation, characterization, and use for plant growth promotion under salt stress, of ACC deaminase-producing halotolerant bacteria derived from coastal soil. J Microbiol Biotechnol 20: 1577-1584.
[Crossref], [Google Scholar]
- Murali M, Singh SB, Gowtham HG, Shilpa N, Prasad M, et al. (2021) Induction of drought tolerance in Pennisetum glaucum by ACC deaminase producing PGPR-Bacillus amyloliquefaciens through antioxidant defense system. Microbiological Research 253: 126891.
[Crossref], [Google Scholar]

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences