ISSN : 2348-1927
Annals of Biological Sciences
The Effects of Ultraviolet Light on Anthocyanin Accumulation in the Adventitious Roots of Sedum wrightii (Crassulaceae)
Stephen F. Austin State University, Texas, USA
Abstract
Several studies have supported the idea that anthocyanin accumulation may be a possible protection mechanism
in plants against DNA damage caused by ultraviolet radiation (UV). This study explored the accumulation of
anthocyanins in the adventitious root tips of Sedum wrightii using the following treatments: UVA, UVA+low UVB,
and UVA+high UVB. Following exposure to UV radiation, samples were analyzed for anthocyanin accumulation
using an ethanol extraction procedure. Using ELISA, additional root samples were analyzed for indicators of DNA
damage: cyclobutane pyrimidine dimers (CPDs) and pyrimidine (6-4) pyrimidone dimers (6-4 PPs). The anthocyanin
concentrations were significantly higher in the UVA + high UVB treatment than the other groups. The ELISA results
showed that a difference occurred between the control and the treatments of UVA, UVA+low UVB and UVA+high
UVB for CPDs and between the control and the UVB treatments for 6-4 PPs. Anthocyanins accumulated with
increased UV exposure. However, additional research is needed to determine the significance of anthocyanins in the
adventitious root tips of S. wrightii.
Keywords
Anthocyanin accumulation, Adventitious roots, Crassulaceae, Sedum wrightii, Ultraviolet radiation
Introduction
Sedum wrightii, a member of Crassulaceae, is a succulent plant that has thick, fleshy leaves arranged in spiraling rosettes around the stem [1]. Leaves easily detach with slight pressure [2]. Clausen [1] states S. wrightii reproduces either sexually through flowers or asexually from detached leaves. Clausen [1] and Gravatt and Taylor [3] report, roots grow from the detached leaflets and eventually become long enough to reach the soil. Sedum wrightii grows primarily in limestone soil and has been reported in Sierra Madre Oriental and adjacent Mexican Plateau, the Davis Mountains in Texas, and on the edge of Edwards Plateau in Texas. The climate is arid and the vegetation includes large desert shrubs and herbs [1]. The focus of this study is on the adventitious root tips of the detached leaflets of S. wrightii where the accumulation of a red-purple pigment was clearly observed to be associated with the root cap and meristematic regions of the root tip (Figures 1 and 2).
Many studies on plants have shown the accumulation of anthocyanins, a member of the flavonoid group [4,5] upon exposure to ultraviolet radiation (UV). Three regions of UV exist: UVA (400-320 nm), UVB (320-290 nm) and UVC (290-200 nm) [6]. Much UVA and little UVB reach the earth’s surface while UVC does not reach the earth’s surface [7]. Anthocyanins have been shown to accumulate in the adventitious roots of Metrosideros excelsa and the leaves of Sedum album upon exposure to the sun [8,9]. The whole roots of maize accumulated anthocyanins upon exposure to UVB [10]. Other studies in leaves, apple skins, and seedlings have also shown an increase in anthocyanin accumulation upon exposure to UVB [11-17]. The first hypothesized result of this study was that the concentration of anthocyanins in the adventitious root tips of S. wrightii would increase upon exposure to increased amounts of UVB. Studies of the effects of UVB on plants have shown morphological effects; physiological effects; developmental effects; increased vulnerability to diseases, genomic instability and alteration in species competition; and molecular effects [7,18-23]. This study focused on the molecular effects of UVB on plants, particularly on DNA damage caused by the induction of cyclobutane pyrimidine dimers (CPDs) and pyrimidine (6-4) pyrimidone dimers (6-4 PPs) [7,23- 26], since cell division occurs in the meristematic region of the adventitious root tips of S. wrightii. Plants minimize DNA damage by DNA repair mechanisms or the accumulation of UVB absorbing compounds, such as anthocyanins [7,27,28]. A study by Takahashi et al. [29] showed pyrimidine dimer concentrations in Centaurea cyanus stem cell cultures would decrease as anthocyanin concentrations increased upon exposure to UVB. The second hypothesized result of this study was that pyrimidine dimer concentrations decreased when anthocyanin concentrations increased in the adventitious root tips of S. wrightii upon exposure to increased amounts of UVB.
Materials And Methods
Collection and growing conditions
Parent plants of S. wrightii were collected from the north side of limestone cliffs along the Devil’s Arm Branch on the edge of Edwards Plateau in Amistad National Park, near Del Rio, TX in February of 2010. These plants were used to provide sample leaves for the experiments. The collected plants were maintained in the Biology Greenhouse of Stephen F. Austin State University through October and then translated to a plant chamber within the greenhouse until needed. About fifteen to thirty leaflets were randomly placed in pots on moist vermiculite in a plant growth chamber for three weeks (14 h photoperiods, 70% RH and day/night temperatures of 20°C/16°C). The pots were placed in trays holding water. Each tray held six to eight pots. The leaflets were placed under filtered fluorescent lights until adventitious roots were well formed, usually within 10-14 days. Mylar-D™ film was used to prevent exposure to low levels of UV produced by the fluorescent lamps.
UV light treatments
After two weeks, the pots were randomly distributed among four different UV light treatments: control, UVA, UVA+low UVB and UVA+high UVB. Mylar-D™ and cellulose acetate films were used to obtain the UV treatments [30]. Mylar film blocked UVB and UVC radiation while cellulose acetate film blocked UVC radiation [30]. Ultraviolet lamps were used as a source for UV radiation. From personal observation of the Devil Arm’s cliff at Amistad National Park, S. wrightii was predicted to receive the most sunlight between 10:00 am and 2:00 pm. Since Stephen F. Austin State University has about the same latitude as Amistad National Park (Nacogdoches 31.5°N, Amistad National Park, 29.5°N), the dosage of UV from sunlight between 10:00 am and 2:00 pm was measured on a summer day (June 2010) using a UV meter (Model: PMA 2100, Solar Light Company, Glenside, PA). Field observations by the authors noted that roots tips made contact with the surface of the soil within 3-5 days of their appearance. The UV dosages were then simulated in the light treatments of the plant growth chamber. The intensity of the UV dosages was altered by adjusting the height of the trays from the source. After a four day period, the roots were sampled by removing tissue from the root tip to one centimeter behind the root tips. Each sample contained 15 to 30 roots. The roots were weighed, divided evenly for anthocyanin extractions and pyrimidine dimer detection and stored at -80°C until needed.
Anthocyanin extraction
The protocol described by Abdel-Aal and Hucl [31] was used for extracting anthocyanins from the roots of S. wrightii. The root tissue was ground with a plastic hand homogenizer (Kimble-Chase, 1.5 mL Pestle and Tube RNase/DNase/ Pyrogen free) and centrifuged for fifteen minutes at 2,100 xg at 4°C. The following changes to the protocol were made. The acidified ethanol (85 EtOH:15 HCl) and S. wrightii root tips mixture was refrigerated for 24 h instead of being shaken. Kuromanin chloride solutions were used to produce a standard curve. Anthocyanin content was determined spectrophotometrically according to procedures published by Abdel-Aal and Hucl [31]. The samples were expressed per fresh weight and per root tip for data analysis.
DNA extraction and pyrimidine dimer detection
The extremely small size of individual roots necessitated the need to pool samples within each replicate (about 435 roots per replicate) for the DNA isolation procedure. The Mini Protocol for Plant tissue of the DNeasy Plant Mini Kit (QIAGEN, Netherlands) was used for extracting DNA from the root samples with the exception that the roots were ground with plastic hand homogenizers rather than the TissueLyser or TissueRuptor. The isolated DNA was quantified via UV absorbance and diluted to 0.2 μg/mL.
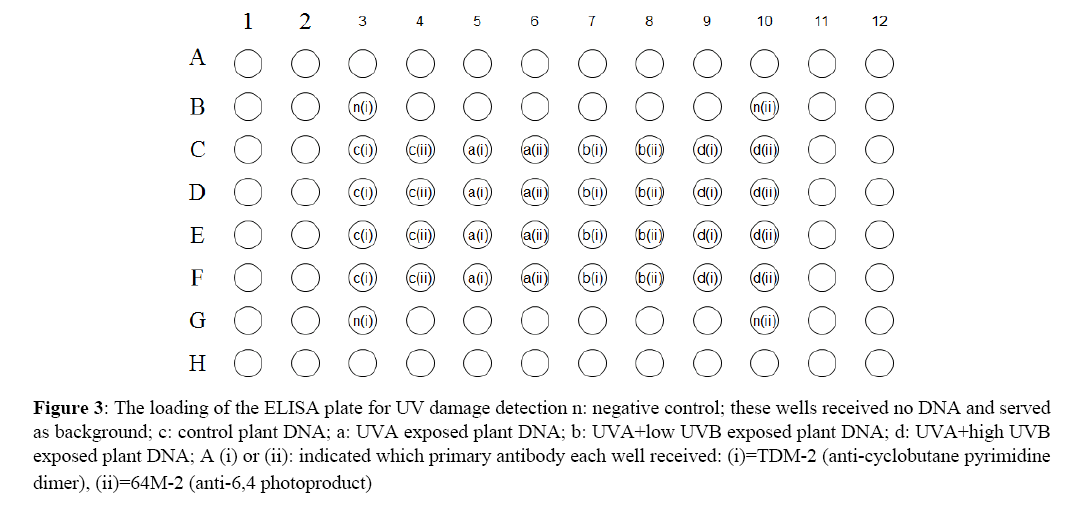
Pyrimidine dimers were quantified with an ELISA based procedure utilizing mouse monoclonal antibodies specific for CPD’s (antibody TDM-2) or the 6-4 PP’s (antibody 64M-2). Both antibodies were purchased from Cosmo Bio Co., Ltd. (Japan). The ELISA plates (96 well) were prepared and loaded with DNA following a protocol provided by Cosmo Bio Ltd. In Figure 3, application of DNA from the four UV treatments and negative controls are shown. The ELISA procedure itself was conducted using Cosmo’s protocol; antibody TDM-2 was used as the primary in two of the negative control wells and four wells each of the four DNA treatments while antibody 64M-2 was used in the other 2 negative control wells and the remaining four wells containing isolated DNA (Figure 3). Colorimetric change produced by the binding of the secondary antibody was measured on an iMark ELISA plate reader at 492 nm and expressed as optical density.
Figure 3: The loading of the ELISA plate for UV damage detection n: negative control; these wells received no DNA and served as background; c: control plant DNA; a: UVA exposed plant DNA; b: UVA+low UVB exposed plant DNA; d: UVA+high UVB exposed plant DNA; A (i) or (ii): indicated which primary antibody each well received: (i)=TDM-2 (anti-cyclobutane pyrimidine dimer), (ii)=64M-2 (anti-6,4 photoproduct)
Statistical analysis
The anthocyanin content per gram of fresh weight (nmol g-1 fw) and per root tip (nmol/root tip) was analyzed with a one-way analysis of variance (ANOVA) using the SAS program (SAS Institute, Cary, NC). When the F statistic had a p-value ≤ 0.05, Tukey’s HSD test and Fisher’s LSD test were used to make comparisons of the averages of the anthocyanin content between treatments.
UV damage quantification
The optical density (OD) average for the four negative control wells was 0.01 OD’s, indicating that any colorimetric change would be due to binding of the primary and secondary antibodies. OD’s for each UV treatment (control, UVA, UVA + low UVB, UVA + high UVB) and each primary antibody, TDM-2 and 64M-2, were calculated for each of the four wells that received the treatment / antibody combination; these four measurements were averaged. If a DNA / UV treatment average OD was greater than control DNA plus 3 standard deviations, it was considered to be significant.
Results
Anthocyanin extraction
Anthocyanin per fresh weight of root tissue showed no significant difference (F0.05(1),3,133=1.63, p=0.1847) between any of the treatments (Table 1). Anthocyanin content on a per root tip basis showed a significant difference (F0.05(1),3,92=4.11, p=0.0087) between the treatments. Tukey’s HSD test showed treatment UVA+high UVB was significantly different from the control (HSD value=0.0167). Treatments UVA and UVA+low UVB, were not significantly different from the control. Treatments UVA, UVA+low UVB and UVA+high UVB were also not significantly different from each other (Table 1). Fisher’s LSD test showed treatment UVA+high UVB was significantly different from the control and UVA+low UVB treatments (LSD value=0.0127; Table 1). Treatment UVA+high UVB was not significantly different from the UVA treatment (Table 1). The UVA treatment was significantly different from the control (LSD value=0.0127) but not significantly different from the UVA+low UVB treatment (Table 1). The UVA+low UVB treatment was not significantly different from the control treatment (Table 1).
| Treatment | Average (nmol g-1 fw) |
Average (nmol anthocyanin/root tip) | |
|---|---|---|---|
| Tukey’s HSD | Fisher’s LSD | ||
| Control | 220.63 | 0.0552 a | 0.0552 a |
| UVA | 200.45 | 0.0688 a, b | 0.0688 b,c |
| UVA+low UVB | 250.49 | 0.0586 a, b | 0.0586 a,b |
| UVA+high UVB | 255.61 | 0.0751 b | 0.0751 c |
Note: Different letters following means, within a column, indicate the treatments that are significantly different from the control using Tukey’s HSD or Fisher’s LSD. Also, no significant differences occurred between the treatments for the nmol g-1 fresh weight (fw) data
Table 1: Mean values for anthocyanin content of S. wrightii root tips
UV damage quantification
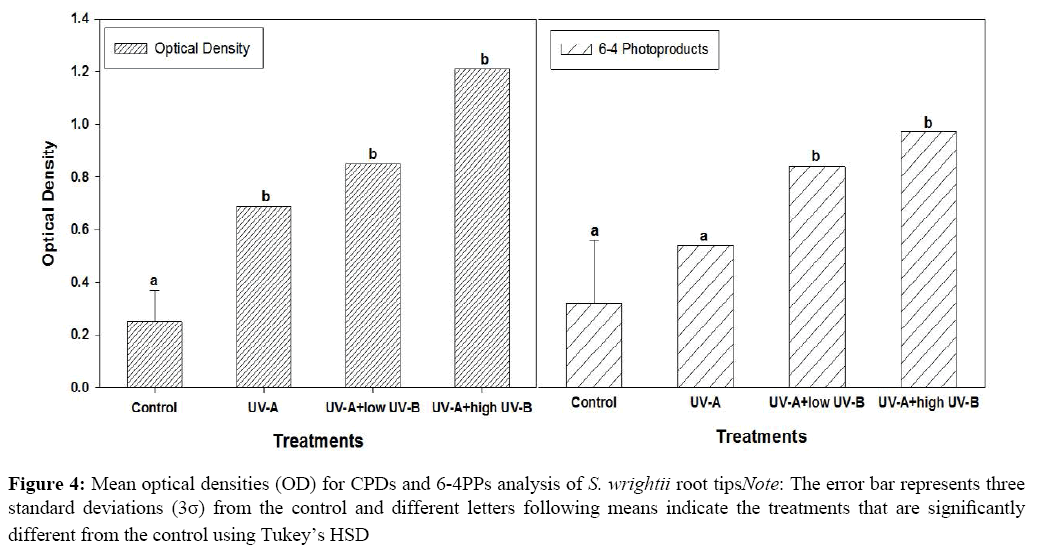
Control DNA treated with TDM-2 or 64M-2 had average OD’s of 0.23 (σ=0.053) and 0.28 (σ=0.1). All UV treatments (UVA, UVA+low UVB, UVA+high UVB) demonstrated significantly higher average OD’s for both primary antibodies (Table 2). With respect to antibody TM-2, while all UV treatments produced significantly higher OD’s than the control DNA, none were significantly different from one another, indicating that all UV treatments were equally good at inducing CPD’s (Figure 3). Antibody 64M-2 produced OD’s significantly higher than that of control DNA across all UV treatments but, additionally, when UVB was added, either high or low, the OD’s were significantly higher than with UVA alone (Figure 3). Thus, UVB exposure added significantly to 6,4-PP formation (Figure 4).
| Treatment | Antibody | |
|---|---|---|
| TDM-2 (anti-cyclobutane dimer) | 64M-2 (anti-6,4 photoproduct) | |
| Control | 0.23 (σ=0.053) | 0.28 (σ=0.1) |
| UVA | 0.69 | 0.50 |
| UVA+low UVB | 0.82 | 0.81 |
| UVA+high UVB | 1.21 | 1.0 |
Table 2: Average optical densities for each DNA/UV/primary antibody combination in the ELISA
Discussion
The first hypothesis of anthocyanins accumulating in the adventitious root tips of S. wrightii upon exposure to UVB was based on the results of recent studies in roots [9,10]. Studies in other plant organs have also supported the hypothesis that anthocyanins accumulate upon exposure to UVB [8,12-17,19,32-34]. The results of the anthocyanin root tip analysis showed a significant difference occurred between the control and UVA+high UVB treatment, supporting the hypothesis. Anthocyanins accumulated significantly more when the adventitious root tips of S. wrightii were exposed to higher amounts of UVB radiation.
The previous studies suggested that in roots anthocyanins functioned as UVB screens [9,10]. Based on previous studies, the second hypothesis of anthocyanin concentrations increasing and pyrimidine dimer concentrations decreasing upon the exposure of the adventitious root tips of S. wrightii to increased amounts of UVB was made. The study by Takahashi et al. [29] on the cells of stems of Centaurea cyanus supported that the presence of anthocyanins reduced dimer formation upon exposure to UVB and UVC radiation. However, a study by Hada et al. [27] argued against the UVB screen hypothesis because increased levels of anthocyanins lowered CPD photorepair in the leaves of purple rice.
The most challenging part of the study was obtaining a detectable amount of DNA from the S. wrightii adventitious root tips. Several procedures were tried for locating the nuclei, including the use of a DAPI staining procedure and a Feulgen staining procedure [35,36]. The observation was made that the root tips of S. wrightii have very few nuclei which appear to be located mainly near the root cap and apical meristem.
Due to the extremely small size of the roots it was necessary to pool all samples within each replicate. Isolation of DNA was accomplished using the DNA Qiagen Kit. Once the DNA was isolated, the pyrimidine dimers were detected using ELISA. The results from ELISA showed treatments UVA, UVA+high UVB and UVA+low UVB were significantly different from the control for CPD’s, but only treatments UVA+high UVB and UVA+low UVB were significantly different from the control for 6-4 PP’s (Figure 4). Note the results of the experiment did not support the hypothesis. Given the small sample size, additional work is needed for the determination of a correlation between anthocyanin accumulation and the formation of pyrimidine dimers in the adventitious root tips of S. wrightii. To account for the potential presence of dimer repair mechanisms, differing UV dosages and sampling times after exposure should be considered.
Conclusion
In conclusion, anthocyanin accumulated significantly more when the adventitious root tips of S. wrightii were exposed to a higher amount of UVB radiation. Data from this study suggests that the presence of anthocyanin did not protect DNA from UV damage. Future research might include identifying the specific anthocyanin and pyrimidine dimer accumulation using TEM techniques, with gold probes, for detecting the dimers in single cells of the adventitious root tips of S. wrightii. Also, comparative studies of anthocyanin accumulation in root tips using Sedum and other related taxa are needed.
Acknowledgement
The authors thank Drs. Josephine Taylor and Michelle Harris of Stephen F. Austin State University; Amistad National Park, and SFA Office of Research and Sponsored Programs.
References
- Clausen, R.T., Cornell University Press, 1975. p.742.
- Gravatt, D.A., Photosynthetica, 2003. 4: p. 449-452.
- Gravatt, D.A. and Taylor, J., SIDA, 2004. 21: p. 943-950.
- Wu, X., et al., J Agric Food Chem, 2006. 54: p. 4069-4075.
- Tanaka, Y., Sasaki, N. and Ohmiya, A., Plant J, 2008. 54: p. 733-749.
- Diffey, B.L., Methods, 2002. 28(1): p. 4-13.
- Frohnmeyer, H. and Staiger, D., Plant Physiol, 2003. 133: p. 1420-1428.
- Bachereau, F., Marigo, G. and Asta, J., Physiol Plant, 1998. 104: p. 203-210.
- Solangaarachchi, S.M. and Gould, K.S., N Z J Bot, 2001. 39: p. 161-166.
- Singh, A.M., Selvi, T. and Sharma, R., J Exp Bot, 1999. 50: p. 1619-1625.
- Mohr, H. and Drumm-Herrel, H., Physiol Plant, 1983. 58: p. 408-414.
- Krizek, D.T., Britz, S.J. and Mirecki, R.M., Physiol Plant, 1998. 103: p. 1-7.
- Mendez, M., Jones, D.G. and Manetas, Y., New Phytologist, 1999. 144: p. 275-282.
- Yang, Z.M., et al., J Environ Sci, 2000. 12: p. 236-240.
- Alexieva, V., et al., Plant Cell Environ, 2001. 24: p. 1337-1344.
- Solovchenko, A. and Schmitz-Eiberger, M., J Exp Bot, 2003. 54: p.1977-1984.
- Mahdavian, K., Ghorbanli, M.K. and Kalantari, M., J Bot, 2008. 32: p. 25-33.
- Jansen, M.A.K., Gaba, V. and Greenberg, B.M., Trends Plant Sci, 1998. 3: p. 131-135.
- Ries, G., et al., Nature, 2000. 406: p. 98-101.
- Bieza, K. and Lois, R., Plant Physiol, 2001. 126: p. 1105-1115.
- Bawhey, C.I., Grant, R.H. and Gao, W., Agric Meteorol, 2003.120: p.161-175.
- Gao, W., et al., J Photochem Photobiol B, 2004. 80: p. 127-131.
- Klisch, M., et al., Aquat Sci, 2005. 67: p. 72-78.
- Stapleton, A.E. and Walbot, V., Plant Physiol, 1994. 105: p. 881-889.
- Quaite, E.F., et al., Plant Cell, 1994. 6: p. 1635-1641.
- Britt, A.B., Annu Rev Plant Physiol Plant Mol Biol, 1996. 108: p. 891-896.
- Hada, H., et al., Plant Cell Environ, 2003. 26: p. 1691-1701.
- Britt, A.B., Photosynth Res, 2004. 81: p. 105-112.
- Takahashi, A., Takeda, K. and Ohnishi, T., Plant Cell Physiol, 1991. 32: p. 541-547.
- Mackenzie, J.L., et al., Genetics, 2005. 171: p. 715-723.
- Abdel-Aal, E.S.M. and Hucl, P., Cereal Chem, 1999. 76: p. 350-354.
- Burger, J. and Edwards, G.E., Plant Cell Physiol, 1996. 37: p. 395-399.
- Chimphango, S.B.M., Musil, C.F. and Dakora, F.D., J Exp Bot, 2003. 54: p. 1771-1784.
- Mori, I.C., J Environ Eng, 2009. 19: p. 21-27.
- Price, H.J., Botanical Gazette, 1980. 141: p. 195-198.
- Al-Adhami, B.H., Appl Environ Microbiol, 2007. 73: p. 947-955.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences