The Effects of Hepatic Ischemia-Reperfusion Injury on Postoperative Cognitive Function in Elderly Patients
Yiqiao Wang, Lina Hao, Pei Gao, Zhenhua Ren and Yuanhai Li*
Department of Anesthesiology, Hospital of Anhui Medical University, Hefei, Anhui, China
- *Corresponding Author:
- Yuanhai L
Department of Anesthesiology
Hospital of Anhui Medical University
218 Jixi Road, Hefei, Anhui, 230022, China
Tel: 86-551-6292-2057
E-mail: liyuanhai-1@163.com
Received date: December 02, 2016; Accepted date: December 26, 2016; Published date: December 30, 2016
Citation: Wang Y, Lina H, Pei G, et al. (2016) The Effects of Hepatic Ischemia-Reperfusion Injury on Postoperative Cognitive Function in Elderly Patients. J Surgery Emerg Med 1:2.
Copyright: © 2016 Wang Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Surgical trauma, such as hepatic ischemiareperfusion (HIR), resulting in the release of peripheral pro-inflammatory cytokines into the brain to affect neurocognitive function, is a prominent risk factor for the development of postoperative cognitive dysfunction (POCD). Methods: Forty patients of partial hepatectomy were divided into two groups: hepatic ischemia-reperfusion group (Group I/R) and control group (Group C). Each group had 20 individuals. Hepatic portal was clamped in the process of partial hepatectomy in the patients of Group I/R, otherwise in the Group C did not block Hepatic portal. Blood sample was collected on the 1th day before surgery and 4th days after surgery respectively, and then the contents of IL-6, TNF-α, S100β protein, AST and ALT were detected by ELISA kit. Neuropsychological tests were performed according Murkin’s standard on the 1th day before surgery and the 4th day, and Z score was counted and used to identify the occurrence of POCD. Result: On the 4th days after surgery, the serum level of IL-6, TNF-α and S100β in the Group I/R patients significantly increased comparing with Group C (P<0.05). Psychological evaluation was performed, and found that Z score was more than 1.96 in 4 patients among Group C, while there were 11 patients in Group I/R. The incidences of POCD in Group I/R were 55%, and obviously higher than 16% in Group C. The incidence of POCD was correlated with serum levels of IL-6, TNF-α and S100β in Group I/R patients. Conclusion: Our results demonstrated that hepatic ischemia-reperfusion in the partial hepatectomy increased the incidence of POCD in elder patients, which was correlated with the elevated levels of IL-6, TNF-α and S100β in peripheral blood.
Keywords
Elderly patients; Hepatic Ischemiareperfusion; Inflammatory factor; POCD
Abbreviation
ALT: Alanine Aminotranferease
AST: Aspartate Aminotransferase
CVP: Central Venous Pressure
ECG: Electrocardiograph
HIRI: hepatic Ischemia-Reperfusion Injury
HIR: Hepatic Ischemia-Reperfusion
HR: Heart Rate
I/R: Ischemia/Reperfusion
MAP: Mean Arterial Pressure
POCD: postoperative Cognitive dysfunction
PAUC: Postanesthesia Care Unit
SpO2: Pulse Oxygen Saturation
TIVA: Total Intravenous Anesthesia
TIC: Target Controlled Infusion
Introduction
Surgical trauma initiates a chain of events that culminate in systemic inflammatory response and multi-organ failure [1]. Liver is one of the most important organs. It is often unavoidable to block the hepatic portal in the liver surgery, such as liver transplantation, partial hepatectomy and other surgical traumas, which inevitably lead to hepatic ischemiareperfusion injury (HIRI), resulting in influences on distant organs [2]. The brain is one of the most vulnerable targets for distant organ damage induced by hepatic ischemia-reperfusion (HIR) [3]. It has been previously reported that HIR, resulting in the release of inflammatory mediators, is a prominent risk factor for the development of postoperative cognitive dysfunction (POCD) [4,5].
Postoperative cognitive dysfunction (POCD) is a complication of the mental system that occurs in postoperative patients. It is manifested in changes in mentality, personality, social activities and cognitive abilities. POCD often occurs in patients undergoing emergency or major surgery, and its clinical features include insanity, anxiety, cognitive decline, impaired memory, decreased comprehension of language and decreased social integration ability [6], which is more common in elderly patients. In clinic, postoperative cognitive dysfunction commonly occurs after HIRI in patients, especially the elderly [7]. Inflammatory response in perioperative period is considered to be a critical factor for the occurrence of POCD. HIR produce a large number of pro-inflammatory cytokines, followed by activation of vascular endothelial cells and induction of the resting and activated T lymphocytes to adhere to brain microvascular endothelial cells [8,9], consequently causing the changes in the permeability of the brain microtubule system and eventually affecting the blood-brain barrier [8].
In addition, S100β protein is a specific biomarker of brain, its content correlates closely with the severity of brain injury and prognosis. Thus, it can be used as a sensitive, specific and reliable biochemical indicator for the assessment of mild and moderate brain injuries [10,11]. In this study, we examined the incidence of POCD in elderly patients undergoing partial hepatectomy with or without blocking the hepatic portal, and investigated the serum levels of IL-6, TNF-α and S100β. We also evaluated the relationship between the incidence of POCD and the levels of these inflammatory factors.
Patients and Methods
Patients and grouping
From April 2015 to May 2016, 40 patients who underwent partial hepatectomy in our hospital, sex-unbiased, were enrolled with 65 to 80 years of age, cardiac function of grade II~III, liver function of grade A, ASA of grade II~III, and with primary education or above. The patients were divided into two groups: non-blocked hepatic portal group (Group C, n=20) and hepatic portal block group (Group I/R, n=20). Those subjects were excluded with any of the following situations: drug addiction, alcoholism, visual and hearing impairment, mental history, or heart and brain vascular disease sequelae. All the qualified participants’ MMSE scores were ≥24 before operation. This study was approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University in accordance with all regulations, and all the patients signed the informed consent of the clinical trial after in-depth communications.
Anesthetic methods
Before anesthetizing, intravenous injection of 0.5 mg of penehyclidine hydrochloride and 10 mg of dexamethasone was given to each patient 30 min pre-operation.
Electrocardiograph (ECG), heart rate (HR), pulse oxygen saturation (SpO2), mean arterial pressure (MAP), central venous pressure (CVP) and nasal temperature were measured in the operating room, and BIS was also monitored to maintain BIS value at 40~60. The induction of anesthesia was performed with 0.03 mg/kg of midazolam, 0.5-1 mg/kg of propofolum, 0.3-0.4 μg/kg of sufentanil, 0.2 mg/kg of cisatracurium, endotracheal intubation and connection to anesthesia apparatus with IPPV mode ventilation, PET CO2 maintained at 35~40 mmHg.
The entire anesthetizing course maintained with total intravenous anesthesia (TIVA) and target controlled infusion (TIC) mode: the serum target concentration of remifentanil was set at 3-5 ng/ml, propofolum at 1-3 μg/ml, intermittent cisatracurium at 0.01-0.02 mg/kg/h. The parameters of anesthesia apparatus were set as follows: ventilation frequency, 10 times/min; tidal volume, 8-10 mg/Kg; inspiration ratio, 1:1.5; oxygen concentration, 0.8-1.0; oxygen flow rate, 1.5 L/min; SpO2>95%. Fluid loss and blood loss during surgery were supplemented accordingly based on the monitoring data and condition changes. Intraoperative temperature of each patient should be maintained with blanket device for insulation to keep the nasal temperature at 36~37°C.
The patients were transferred to resuscitation room for intensive care and observation after surgery, when the patients were fully awake, the evaluation of extubation conditions was carried out and the tracheal catheter was removed in line with extubation criteria. The patients’ dwelling time of post-anesthesia care unit (PAUC) and extubation time were recorded (starting from the end of surgery). Postoperative intravenous analgesia pump was used with sufentanil at a concentration of 0.05 μg/kg/h for analgesia.
Intraoperative Monitoring and Indicators Record
Electrocardiograph (ECG), heart rate (HR), pulse oxygen saturation (SpO2), mean arterial pressure (MAP), central venous pressure (CVP) and nasal temperature were monitored for each patient in the operating room, and after surgery the patients were transferred to resuscitation room for intensive care and observation. The patient’s dwelling/duration time of PAUC and extubation time were recorded (starting from the end of surgery) by a specially-trained investigator.
Surgery time (starting from skin incision to the end of suturing) was also documented. MAP, SpO2, HR, body temperature were recorded for both groups at the following 7 time points, respectively: for Group I/R, before induction of anesthesia (T1), after induction (T2), after tracheal intubation (T3), before hepatic portal block (T4), 10 min after blocking (T5), block removal (T6), and 30 min after block removal (T7); for group C, correspondingly, T4 was defined at ‘before hepatectomy’, T5 at ‘10 min after the beginning of hepatectomy’, T6 at ‘end of keratectomy’, and T7 at ‘30 min after hepatectomy’.
Cognitive Function Assessment and POCD Measurement Methods
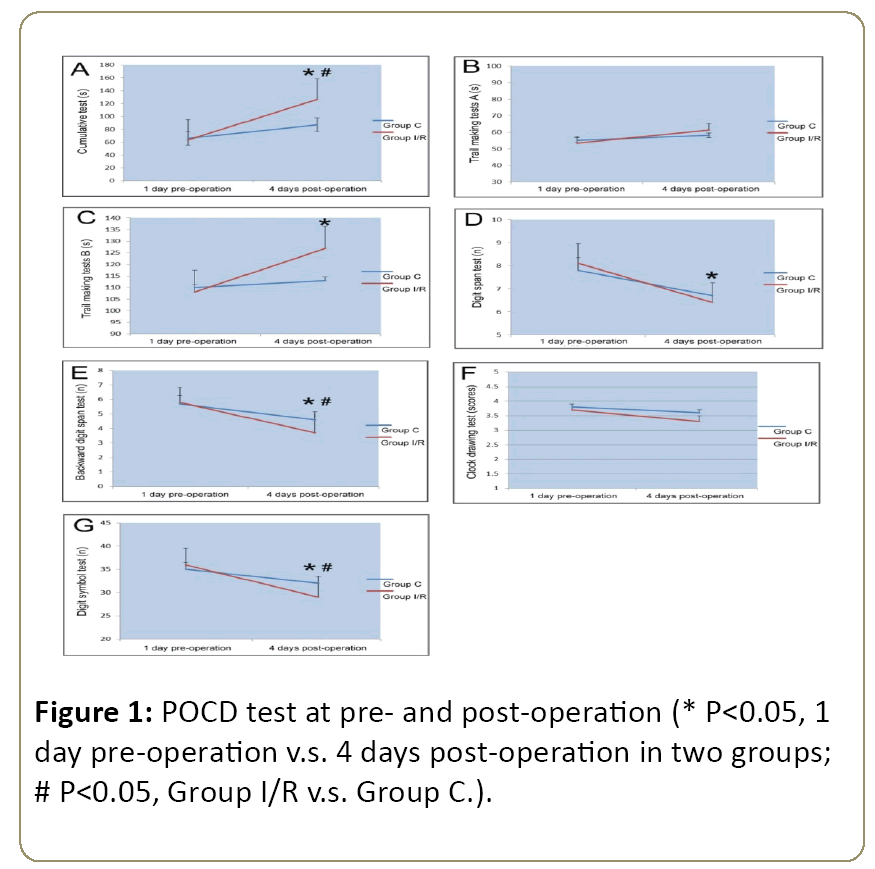
Postoperative cognitive dysfunction (POCD) evaluation was conducted with the testing assays recommended by Murkin et al., at 1 day pre-operation and 4 days post-operation [11]. Briefly, seven testing items were included as follows: cumulative test, trail making tests A and B, digit span test, backward digit span test, clock drawing test and digit symbol test. Before these tests, 160 healthy volunteers were selected to calculate the baseline and practice effect values, respectively.
According to Murkin’s calculation formula: Z≈[absolute value of (postoperative score-preoperative score)-practice effect value]÷standard deviation of the baseline value, POCD diagnostic criterion is defined: if two single Z values (or composite Z value) are greater than or equal to 1.96, then the postoperative cognitive dysfunction is confirmed.
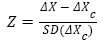
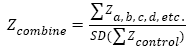
Note: X, the absolute value difference between the test value 4 days post-operation and the test value 1 day preoperation; Xc, the value difference between the baseline values tested twice from healthy control; SD (Xc), standard deviation of Xc.
Inflammatory Factors, AST and ALT Analysis
Venous blood samples of limosis were collected from each subject in the mornings on 1 day before operation and 4 days after operation. Blood samples were placed at room temperature for 2 h, and then centrifuged at 3500 rpm/min for 10 min. Serum was collected immediately and stored at -40°C. Serum IL-6 and TNF-α were determined via commercial ELISA Kits (Wuhan Boster Biological Engineering Co., Ltd., China) in accordance with the manufacturer’s instructions. The detection ranges of the two cytokines were ≤15.6 ng/L and ≤4.69 ng/L, respectively. In addition, the blood samples were harvested before operation, 30 min after operation and 6 h after operation for serum S100β protein detection through ELISA (Beijing Bomais Technology Development Co., Ltd., China). Lastly, serum alanine aminotranferease (ALT) and aspartate aminotransferase (AST) were also measured by an automatic biochemical detector.
Statistical analysis
Statistical analysis was performed using SPSS 15.0, and all measured data were presented as the means ± SD. Vital sign data were analyzed by variance analysis. The level of significant differences in group means was determined by the Student's t-test for parametric data sets. Counting data were compared using the X2 test. Pearson’s partial correlation analyses were also performed between cognitive function scores and serum levels of IL-6, TNF-α and S100β, controlling for general status of patients, to adjust for the possible effects of sex, age, body weight, surgery time and etc. A P value of <0.05 was considered significant in all analyses.
Results
General status of patients
40 patients undergoing partial hepatectomy were enrolled in this study, they were divided into two groups: non-blocked hepatic portal group (Group C) and hepatic portal block group (Group I/R). General status of patients were analyzed, and there were no significant differences in sex ratio, age, body weight, surgery time, PACU dwelling time and tracheal extubation time between two groups (P>0.05, Table 1).
| Group | Sex (M/F) | Age (Y) | Body weight (kg) | Surgery time (min) | PACU dwelling time (min) | Extubation time(min) | |
|---|---|---|---|---|---|---|---|
| Group C | 12-Aug | 70.00 ± 4.00 | 61.30 ± 8.00 | 142.20 ± 46.20 | 53.40 ± 14.10 | 31.40 ± 10.60 | |
| Group I/R | 13-Jul | 70.50 ± 5.00 | 66.00 ± 9.00 | 134.40 ± 31.00 | 55.70 ± 16.40 | 29.30 ± 9.70 |
Table 1: The general status of patients.
Intraoperative vital signs
The vital signs of patients, including Mean Arterial Pressure (MAP), pulse oxygen saturation (SpO2), body temperature and heart rate (HR), were monitored during surgery.
The data of vital signs were recorded at the indicated 7 time points (T1-T7), respectively, as follows: before induction of anesthesia (T1), after induction (T2), after tracheal intubation (T3), before hepatic portal block (T4), 10 min after blocking (T5), block removal (T6), and 30 min after block removal (T7). There were no statistically significant differences between two groups in MAP, SpO2, body temperature and HR (P>0.05, Table 2).
| Vital signs | Group | T1 | T2 | T3 | T4 | T5 | T6 | T7 |
|---|---|---|---|---|---|---|---|---|
| MAP (mmHg) | Group C | 96.92 ± 11.46 | 72.60 ± 8.12 | 91.14 ± 16.23 | 97.81 ± 13.56 | 93.67 ± 8.66 | 91.23 ± 8.72 | 93.27 ± 9.48 |
| Group I/R | 95.94 ± 10.47 | 72.26 ± 8.62 | 92.40 ± 11.16 | 95.20 ± 12.85 | 94.40 ± 12.47 | 91.47 ± 12.76 | 92.40 ± 8.78 | |
| SpO2 (%) | Group C | 99.01 ± 1.31 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| Group I/R | 98.82 ± 1.39 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | |
| T (°C) | Group C | 36.85 ± 0.21 | 36.85 ± 0.18 | 36.84 ± 0.17 | 36.63 ± 0.25 | 36.56 ± 0.24 | 36.43 ± 0.18 | 36.23 ± 0.19 |
| Group I/R | 36.72 ± 0.25 | 36.78 ± 0.26 | 36.67 ± 0.29 | 36.67 ± 0.24 | 36.52 ± 0.25 | 36.42 ± 0.20 | 36.29 ± 0.22 | |
| HR (bpm) | Group C | 71.82 ± 13.27 | 69.83 ± 12.62 | 82.29 ± 13.85 | 82.76 ± 10.26 | 84.31 ± 11.74 | 83.62 ± 11.21 | 84.84 ± 12.16 |
| Group I/R | 76.81 ± 9.35 | 69.16 ± 7.89 | 79.09 ± 11.42 | 83.24 ± 11.46 | 86.93 ± 11.86 | 85.28 ± 12.95 | 78.73 ± 13.64 |
Table 2: MAP, SpO2, T and HR at different time points.
Comparison of POCD Test Results
According to Murkin et al., testing assays [11], the two groups of patients received the following tests, including cumulative test, trail making tests A and B, digit span test, backward digit span test, clock drawing test and digit symbol test, two times at 1 day pre-operation and 4 days postoperation. The results showed that the cumulative test time (Figure 1A) and trail making test B time (Figure 1C) at 4 days postoperation increased significantly compared with those at 1 day pre-operation in Group I/R; otherwise, the digit span test time (Figure 1D), backward digit span test time (Figure 1E) and digit symbol test time (Figure 1G) markedly decreased. Comparing with Group C at 4 days post-operation, the cumulative test time (Figure 1A) and trail making test B time (Figure 1C) remarkably increased, while the backward digit span test time (Figure 1E) and digit symbol test time (Figure 1G) decreased evidently in the Group I/R.
According to the previous standard as described by Murkin et al. [11] Postoperative cognitive dysfunction (POCD) was conducted with the following testing assays at 1 day preoperation and 4 days post-operation. Seven testing items were included as follows: cumulative test, trail making tests A and B, digit span test, backward digit span test, clock drawing test and digit symbol test. Before these tests, 160 healthy volunteers were selected to calculate the baseline and practice effect values, respectively (Figure 1).
Then we analyzed the difference of POCD scores between two groups, and the data indicated that there were significant differences in the accumulative test, trail making test B, backward digit span test and digit symbol test times at 4 days post-operation between the Group I/R and Group C (P<0.05). However, it did not differ significantly among the other tests (P>0.05) (Table 3).
| Item | Group | 1 Day pre-operation | 4 Days post-operation | Difference |
|---|---|---|---|---|
| Cumulative test (s) | Group C | 65.7 ± 19.3 | 87.2 ± 34.1 | 12.1 ± 6.2 |
| Group I/R | 63.4 ± 20.2 | 126 ± 37.6 | 62 ± 14.5 | |
| Trail making test A (s) | Group C | 55.7 ± 9.1 | 58.2 ± 4.4 | 4.7 ± 8.5 |
| Group I/R | 54.6 ± 8.5 | 60.2 ± 7.6 | 4.1 ± 6.3 | |
| Trail making test B (s) | Group C | 113 ± 32 | 113 ± 27 | -0.3 ± 19.8 |
| Group I/R | 108 ± 29 | 127 ± 45 | 20.5 ± 24.3 | |
| Digit span test (n) | Group C | 7.8 ± 1.2 | 6.7 ± 1.3 | -1.0 ± 0.9 |
| Group I/R | 8.1 ± 1.1 | 6.9 ± 1.6 | -1.3 ± 0.8 | |
| Backward digit span test (n) | Group C | 5.7 ± 1.2 | 4.4 ± 1.5 | -1.1 ± 0.6 |
| Group I/R | 5.8 ± 1.3 | 3.8 ± 1.2 | -1.9 ± 0.2 | |
| Clock drawing test (scores) | Group C | 3.5 ± 0.6 | 3.8 ± 0.5 | 0.3 ± 0.5 |
| Group I/R | 3.7 ± 0.5 | 3.5 ± 0.6 | 0.1 ± 0.4 | |
| Digit symbol test (n) | Group C | 35 ± 8 | 32 ± 11 | -4.0 ± 5.3 |
| Group I/R | 36 ± 9 | 29 ± 10 | 1.6 ± 7.8 |
Table 3: The POCD scores pre- and post-operation.
According to the standard Murkin’s Z scoring system [11], In this study, there were 4 cases in Group C and 11 cases in Group I/R, whose individual Z scores were greater than 1.96. The incidence of POCD were 16% and 55% in Group C and Group I/R patients, respectively, suggesting that POCD in Group I/R patients occurs more common than in Group C (P<0.05).
The Serum Levels of IL-6, TNF-Α and S100β
Blood samples were collected from each subject on 1 day pre-operation and 4 days post-operation. The serum levels of IL-6, TNF-α and S100β were measured by ELISA.
As shown in Table 4, the amounts of serum IL-6, TNF-α and S100β in the Group I/R were all higher than those in the Group C at 4 days post-operation (P<0.05).
| Group | Test time | IL-6 (ng/L) | TNF-α (μg/L) | S100β (ng/L) |
|---|---|---|---|---|
| Group C | Day 1 pre-op | 175.26 ± 31.47 | 2.96 ± 0.32 | 478 ± 148 |
| Day 4 post-op | 267.81 ± 52.63a,b | 4.17 ± 0.63a,b | 607 ± 189a,b | |
| Group I/R | Day 1 pre-op | 182.26 ± 38.15 | 3.09 ± 0.41 | 475 ± 154 |
| Day 4 post-op | 353.29 ± 61.46a,b | 11.35 ± 1.27a,b | 774 ± 202a,b |
Note: aP<0.05, compared with 1 day pre-operation; bP<0.05, compared with Group C on 4 days post-operation. pre-op: pre-operation; post-op: post-operation.
Table 4: The serum levels of IL-6, TNF-α and S100β.
In contrast to pre-operation, the serum alanine aminotranferease (ALT) and aspartate aminotransferase (AST) increased in both groups, and peaked at 4 days after operation. Notably, the levels of serum AST and ALT in the Group I/R were significantly higher than those in Group C (P<0.05, Table 5).
| Item | Group | 1 day pre-op | 3 days post-op | 4 days post-op | 5 days post-op | 7 days post-op |
|---|---|---|---|---|---|---|
| AST (U/L) | Group C | 76 ± 32 | 241 ± 102* | 307 ± 136* | 235 ± 89* | 102 ± 64 |
| Group I/R | 82 ± 38 | 778 ± 219*# | 867 ± 214*# | 687 ± 136*# | 131 ± 72 | |
| ALT (U/L) | Group C | 68 ± 27 | 171 ± 84* | 262 ± 125* | 186 ± 63* | 91 ± 35 |
| Group I/R | 73 ± 32 | 532 ± 116*# | 662 ± 129*# | 297 ± 96*# | 105 ± 61 |
Note: *P<0.05, compared with 1 day pre-operation; #P<0.05, compared with Group C. pre-op: pre-operation; post-op: post-operation.
Table 5: The serum levels of AST and ALT.
Partial Correlation Analysis for the Incidence of POCD and the Serum Levels of IL-6, TNF-Α and S100β
We next analyzed the correlation between the incidence of POCD and the serum levels of IL-6, TNF-α and S100β in both two group patients. The increased serum levels of IL-6, TNF-α and S100β in Group I/R was markedly correlated with the incidence of POCD at 4 day after operation (P<0.05, Table 6).
| Item | r value | P value |
|---|---|---|
| IL-6 | 0.812 | 0.021 |
| TNF-α | 0.931 | 0.004 |
| S100β | 0.875 | 0.015 |
Table 6: Partial correlation analysis for POCD incidence and the levels of serum IL-6, TNF-α and S100β.
Discussion
The incidence of postoperative cognitive dysfunction (POCD) is associated with anesthesia methods, age, types of surgery and others. The difference of age and the degree of operation invasion affect the cognitive function of the postoperative patients. POCD is prone to occur with age and more common in the postoperative patients with higher degree of operation invasion [12,13]. One of the reasons for the high incidence of POCD in elderly patients may be due in part to the gradually decreased functions of the central nervous system in elderly patients with age, which likely becomes difficult to counteract the massive stress generated during anesthesia and surgery. During the perioperative period, a large number of inflammatory factors (e.g., cytokines) will be released, and the neurophysiological and neurotransmission functions of the elderly will get damaged, leading to continuous postoperative cognitive impairment [14]. Hepatic ischemia-reperfusion injury is usually able to induce a large number of pro-inflammatory cytokines. It evidenced that upon peripheral inflammation, multiple inflammatory factors such as TNF-α, IL-6, IFN-ɤ, CD40L, LPS can lead to the activation of vascular endothelial cells and induction of the resting and activated T lymphocytes to adhere to brain microvascular endothelial cells [15], consequently causing the changes in the permeability of the brain microtubule system and therefore affecting the blood-brain barrier.
In this study, the two groups of patients all had increased expressions of TNF-α and IL-6 4 days after surgery, however, the ischemia-reperfusion group increased more pronouncedly. Ishida et al., [16] found that the greater the trauma degree of surgery was, the higher the incidence of disabilities related to daily life would be, suggesting the extensive impacts of surgery on cognitive functions and trauma degree. The serum level of S100β protein can be regarded as a diagnostic indicator for brain damage, which is correlated with the degree of brain injury. It has reported that elderly patients have different degrees of brain injury after surgery [17].
That the neuropsychological tests were performed at 4 days post- operation was taking into consideration as follows: (1) psychological tests would not be expected to conduct within 72 hours to avoid the effects of any anesthetics on cognitive functions in this experiment; (2) the patients felt painful within 48 hours after surgery, which may affect their mood, psychological state, so in order to reduce artificial errors, the tests should be done when patients got pain relief after subjective evaluation; (3) the degree of liver function impairment may affect cognitive function, neuropsychological evaluation should be performed when liver function was damaged the most severely, the severity of liver injury can be evaluated indirectly by serum AST and ALT levels. On the fourth day after surgery, the serum levels of AST and ALT in two groups reached the peak, and then decreased gradually. Similarly, in order to reduce the practice effect errors as a result of multiple evaluations which made the patients became more skillful at the testing, according to Murkin’s Z score standard [11], only one evaluation test should be carried out in the short term after surgery, and dynamic evaluation during hospitalization cannot be fulfilled.
In the present study, seven psychiatric tests were followed to assess the cognitive functions of the elderly patients in terms of memory, orientation, continuous attention, psychomotor speed, spatial thinking transformation, and etc. The results demonstrated that the scores of multiple tests were correlated with the degree of liver injury, and released cytokines.
In summary, the ischemic-reperfusion injury in elderly patients increased the incidence of POCD, companied with the increased serum levels of IL-6, TNF-α and S100β. These inflammatory factors correlated significantly with the incidence of POCD. Therefore, post-operative detection of serum IL-6, TNF-α and S100β levels would indirectly reflect or predict the occurrence of POCD in post-anesthesia patients, which should be beneficial for early detection and intervention, to prevent long-term cognitive function impairment.
Acknowledgement
This research is supported by grants from the Research Fund for the Doctoral Program of Higher Education of China (20133420110009). It is also supported in part by the National Natural Science Foundation of China (81372693) and the Anhui Natural Science Foundation (1308085MH119).
Conflict of Interest
The authors declare that they have no conflicts of interest with the contents of this article.
References
- Colletti LM, Kunkel SL, Walz A, Burdick MD, Kunkel RG, et al. (1996) The role of cytokine networks in the local liver injury following hepatic ischemia/reperfusion in the rat. Hepatology 23: 506-514.
- Tu FP, Li JX, Li Q, Wang J (2016) Effects of hydrogen sulfide on cognitive dysfunction and NR2B in rats. J Surg Res 205: 426-431.
- Pelinka LE, Harada N, Szalay L, Jafarmadar M, Redl H, et al. (2004) Release of S100B differs during ischemia and reperfusion of the liver, the gut, and the kidney in rats. Shock 21: 72-76.
- Rosczyk HA, Sparkman NL, Johnson RW (2008) Neuroinflammation and cognitive function in aged mice following minor surgery. Exp Gerontol 43: 840-846.
- Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, et al. (2010) Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci, USA 107: 20518-20522.
- Wang YQ, Wu WW, Wang LK, Chen K, Li YH (2014) Influence of hepatic ischemia-reperfusion on postoperative spatial cognitive function in mice. Genet Mol Res 13: 5767-5777.
- Tu F, Li J, Wang J, Li Q, Chu W (2016) Hydrogen sulfide protects against cognitive impairment induced by hepatic ischemia and reperfusion via attenuating neuroinflammation. Exp Biol Med 241: 636-643.
- Anvar NE, Saliminejad K, Ohadi M, Kamali K, Daneshmand P, et al. (2015) Association between polymorphisms in Interleukin-16 gene and risk of late-onset Alzheimer's disease. J Neurol Sci 358: 324-327.
- Plaschke K, Hauth S, Jansen C, Bruckner T, Schramm C, et al. (2013) The influence of preoperative serum anticholinergic activity and other risk factors for the development of postoperative cognitive dysfunction after cardiac surgery. J Thorac Cardiovasc Surg 145: 805-811.
- Li JZ, Li XZ, Wang XM, Wang MS, Yu HF, et al. (2013) Effects of parecoxib sodium analgesia on serum concentrations of neuron-specific enolase and S-100β and postoperative cognitive function of elderly patients undergoing acute replacement of femoral head. Zhong hua Yi Xue Za Zhi 93: 2152-2154.
- Murkin JM, Newman SP, Stump DA, Blumenthal JA (1995) Statement of consensus on assessment of neurobehavioral outcomes after cardiac surgery. Ann Thorac Surg 59: 1289-1295.
- Tan CB, Ng J, Jeganathan R, Kawai F, Pan CX, et al. (2015) Cognitive changes after surgery in the elderly: does minimally invasive surgery influence the incidence of postoperative cognitive changes compared to open colon surgery. Dement Geriatr Cogn Disord 39: 125-131.
- Shoair OA, Grasso Ii MP, Lahaye LA, Daniel R, Biddle CJ, et al. (2015) Incidence and risk factors for postoperative cognitive dysfunction in older adults undergoing major noncardiac surgery: A prospective study. J Anaesthesiol Clin Pharmacol 31: 30-36.
- Kong F, Chen S, Cheng Y, Ma L, Lu H, et al. (2013) Minocycline attenuates cognitive impairment induced by isoflurane anesthesia in aged rats. PLoS One 8: e61385.
- Athanasopoulos P, Mastoraki A, Papalois A, Nastos C, Kondi-Pafiti A, et al. (2016) Expression of inflammatory and regenerative genes in a model of liver ischemia- reperfusion and partial hepatectomy. J Invest Surg 29: 67-73.
- Ishida K, Yamashita A, Uchida M, Matsumoto M (2014) Development of postoperative cognitive dysfunction following major vascular surgery. Masui 63: 1211-1218.
- Zhao GL, Ding M (2013) Correlationship between postoperative cognitive dysfunction and time course of serum neuron-specific enolase, S100βand β-amyoid peptide in elderly patient. J Clin Anesthesiol 29: 979-982.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences