The Determining Role of Silicon in Bone Formation: Review and Clinical Results
Alberghini Maltoni Andrea and Giovanni Suffritti*
Department of Research and Development, Ghimas, Casalecchio de Reno, Bologna, Italy
- *Corresponding Author:
- Giovanni Suffritti
Department of Research and Development
Ghimas via Domenico Cimarosa
Casalecchio Di Reno, Bologna, Italy
Tel: +39 051 575353
E-mail: giovanni.suffritti@ghimas.it
Received Date: March 03, 2018; Accepted Date: March 23, 2018; Published Date: March 30, 2018
Citation: Andrea AM, Suffritti G (2018) The Determining Role of Silicon in Bone Formation: Review and Clinical Results. J Clin Med Ther. 3:5.
Short Communication
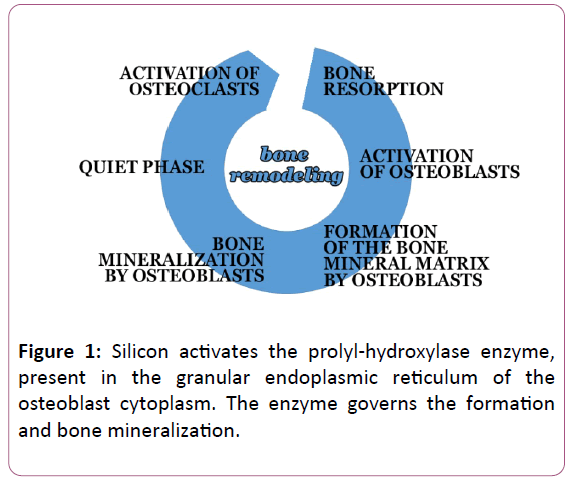
Bone tissue consists of cells that reabsorb bone (osteoclasts) and cells that form and mineralize bone (osteoblasts). Osteoblasts produce collagen and hydroxyapatite, which together are the constituents of the mineral part of the bone (Figure 1).
The bone tissue is subjected to a continuous remodeling process, summarized below:
This process is always present, for a lifetime
• with a positive balance up to around 30 years
• with negative balance subsequently (with a 2% drop every year)
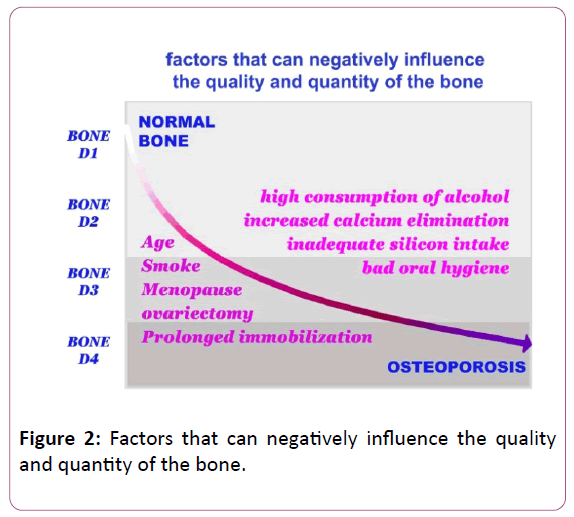
This physiological decline mainly affects calcium and can be increased by other favourable factors:
• Poor calcium and silicon diet or excessive sodium intake.
• Sedentary life.
• High and prolonged intake of alcohol.
• High and protracted coffee intake.
• Cigarette smoke.
• The reduction of gonadal function that occurs in menopause involves a further increase in calcium loss (osteoporosis).
• Even bad oral hygiene can adversely affect bone remodeling at the oral level.
Silicon activates and ensures the normal and physiological synthesis of bone tissue
Common experience indicated that the diet enriched with Equisetum arvense had positive effects on the growth of farmed animals. So starting from the 60s it began to study which was the reason and it was highlighted that silicon was responsible for this result. In the cartilaginous and bone tissues there was a greater amount of silicon in the phase of growth compared to that present in the phases of quiescence and stability [1-5].
In all the cells involved in the development of the structural tissues (dermal matrix, cartilage and bone) is present the prolyl-hydroxylase enzyme which is determinant for their specific function. Through the activity of prolyl-hydroxylase the fibroblasts produce collagen for the intracellular matrix, the chondroblasts form the cartilage and the osteoblasts are able to produce collagen and hydroxyapatite, which are at the base of the formation of the mineral part of the bone tissue [6].
Silicon, in fact, is indispensable in the early stages of collagen synthesis, conditioned by the activity of the enzyme prolyl-hydroxylase, silicon-dependent enzyme, present in the endoplasmic reticulum of cells that synthesize collagen (fibroblasts, chondroblasts and osteoblasts) [7-8].
Other studies have also documented that the addition of silicon from Equisetum arvense in the diet is able to restore and enhance the activity and functionality of these cells, improving any pathological conditions [9-11].
Consequently, the use of silicon supplementation can constitute a preventive and curative treatment, in all quantitative alterations of bone mass (osteoporosis, osteoarthritic or post-traumatic bone degeneration, bone fractures, orthopaedic and dental surgery, and guided bone regeneration): in fact, silicon promotes and activates, qualitatively and quantitatively, the bone remodelling processes [12-17].
In particular, the study by Mattioli-Belmonte et al. [18] has documented in vitro the efficacy of OSTEOSIL Calcium in improving the viability and metabolic function of osteoblasts.
OSTEOSIL Calcium (Ghimas SpA, Casalecchio di Reno- Bologna, Italy) is a dietary supplement based on calcium and silicon present in Equisetum arvense.
Osteoblasts were cultured with OSTEOSIL Calcium in an increasing concentration of 2.5 μg/ml to 10 μg/ml and it was observed at SEM that the cell morphology was directly proportional to the concentration of OSTEOSIL Calcium. In cell cultures treated with the highest concentration, cell morphology was the best (good adhesion, cell viability and absence of degenerative aspects).
The concentrations between 2.5 and 10 μg/ml, achieved by the recommended dosage of OSTEOSIL Calcium, thus represent the optimal treatment for a balanced supply of silicon and calcium, even for prolonged periods of administration.
The EDAX micro analytic investigation also confirms that silicon and calcium administered with OSTEOSIL Calcium are usefully used by osteoblasts in their metabolism aimed at bone production.
Silicon activates prolyl-hydroxylase, which synthesizes collagen, a precursor of bone
Silicon, in an orally absorbable form, optimizes the activity of the silicon-dependent prolyl-hydroxylase enzyme, enzyme present in the endoplasmic reticulum of the osteoblast, which synthesizes the collagen, precursor of the bone, more rapidly and copiously (Figure 2).
The osteoblasts activated by the silicon are able to make better use of the blood calcium, which would otherwise be clinically very useful.
It is known that calcium, as it ages, undergoes its physiological decline, which often increases due to the concomitant presence of unfavourable conditions.
The available calcium or that taken with the diet, however, in the presence of silicon instead becomes more bioavailable and used for the phases of bone remodelling.
The functional synergism between osteoblasts and osteocytes allows to strengthen the formation of new bone and to improve its mineralization, obtaining a bone, which in implantology makes the implant stable.
Greater quantity and better bone quality in implantology with OSTEOSIL Calcium
This is the rationale underlying the clinical outcome observed after supplementation with OSTEOSIL Calcium in 124 subjects (45 females and 79 males, age 61.4 + 14.6 years, range 41-79 years) who needed an implant surgery.
The basic CT scan showed bone D1 in 15 cases, bone D2 in 37 cases, bone D3 in 54 cases and bone D4 in 18 cases.
After supplementation of OSTEOSIL Calcium to the daily dosage of two tablets for three months, the implant site showed an optimal bone for both quality and quantity during implant insertion, ensuring better implant stability in all cases (Table 1).
| CT (HU) | Micro CT (BV%) | |||
|---|---|---|---|---|
| Before | After | Before | After | |
| Num. | 124 | 124 | 124 | 124 |
| mean | 728 | 741 | 53,62 | 54,33 |
| SD | 201 | 204 | 16,31 | 16,59 |
| es | 18 | 18 | 1,46 | 1,49 |
| min | 391 | 401 | 30,14 | 30,54 |
| max | 1100 | 1123 | 81,60 | 83,03 |
| var.% | 1,82% | 1,32% | ||
Table 1: Variation of CT scan values before and after supplementation with OSTEOSIL Calcium. Descriptive statistical analysis (mean, SD, ES, minimum and maximum value). The density of bone was first measured in Hounsfield units (HU) using computed tomography (CT) and also in bone volume % (BV%) using micro-CT scan. The percentage change between before and after is also indicated.
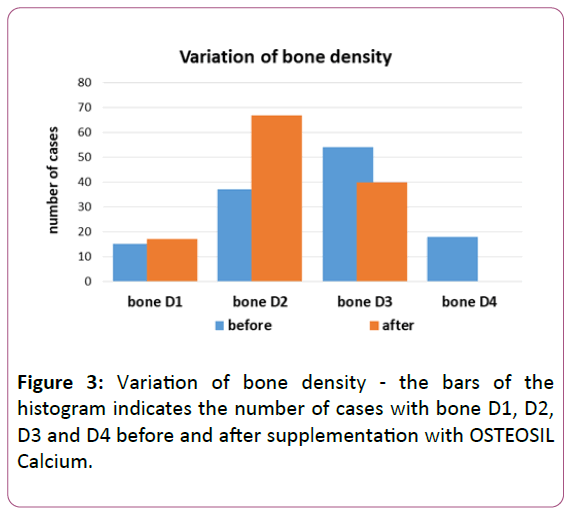
The CT scan performed before implant insertion indicated a sharp increase in bone density (about 1.5% improvement in three months of OSTEOSIL Calcium supplementation): bone D1 in 17 cases, bone D2 in 67 cases and bone D3 in 40 cases, with disappearance of 18 cases with bone D4 (P <0.001 at the Mann Whitney U test) (Figure 3).
The figure indicates how bone D1 is increased in two cases (from 15 to 17); bone D2 in 30 cases (from 37 to 67). This shift towards a better bone quality is counterbalanced by the decrease in cases with less dense bone: of 14 cases with bone D3 (from 54 to 40) and with the total absence of cases with bone D4.
In implant surgery, it will thus be easy to find during supplementation with OSTEOSIL Calcium that the bone of the implant site is more hard and compact.
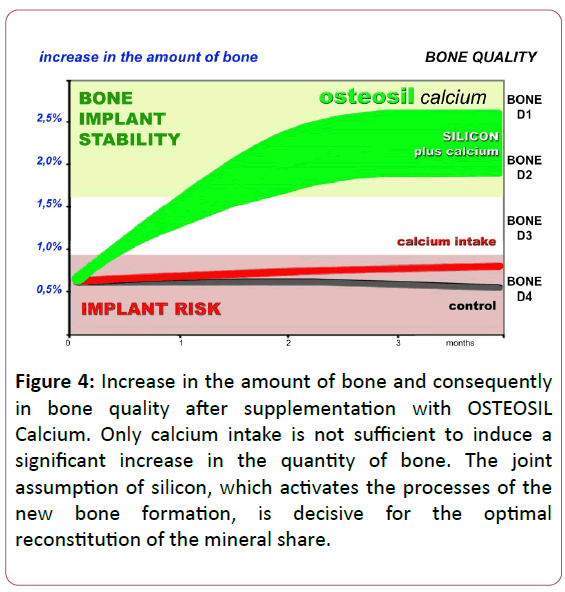
Silicon and calcium thus favour better calcification and a quicker mineralization of bone tissue, which in implantology results in faster bone formation (Figure 4).
Figure 4: Increase in the amount of bone and consequently in bone quality after supplementation with OSTEOSIL Calcium. Only calcium intake is not sufficient to induce a significant increase in the quantity of bone. The joint assumption of silicon, which activates the processes of the new bone formation, is decisive for the optimal reconstitution of the mineral share.
It follows the logical possibility of being able to "load" the plants in shorter times with great consequent advantages both with the technique of immediate loading and in that of the early load, obtaining, in addition, an increase in the density of the newly formed bone.
References
- Monceaux RH (1960) Le silicium: étude biologique et pharmacologique. Prod Pharm 15: 99.
- Seaborn CD, Nielsen FH (1994) Effects of germanium and silicon on bone mineralization. Biol Trace Elem Res 42: 151-164.
- Kitsugi T, Nakamura T, Oka M, Cho SB, Mijaji F, et al. (1995) Bone-bonding behavior of three heat-treated silica gels implanted in mature rabbit bone. Calcif Tissue Int 57: 155-160.
- Eisinger J, Clairet D (1993) Effects of silicon, fluoride, etidronate and magnesium on bone mineral density: a retrospective study. Magnes Res 6: 247-249.
- Hott M, de Pollak C, Modrowski D, Marie PJ (1993) Short-term effects of organic silicon on trabecular bone in mature ovariectomized rats. Calcif Tissue Int 53: 174-179.
- Reffitt DM, Ogston N, Jugdaohsingh R, Cheung HF, Evans BA, et al. (2003) Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone 32: 127-35.
- Betti V (1990) Effetti dell’estratto secco titolato di Equisetum arvense sull’accrescimento dei denti e delle ossa lunghe nel ratto. Attualità Dentale suppl 7: 1.
- Kotwal SD, Badole SR (2016) Anabolic therapy with Equisetum arvense along with bone mineralising nutrients in ovariectomized rat model of osteoporosis. Indian J Pharmacol 48: 312-315.
- Dong M, Jiao G, Liu H, Wu W, Li S, et al. (2016) Biological Silicon Stimulates Collagen Type 1 and Osteocalcin Synthesis in Human Osteoblast-Like Cells Through the BMP-2/Smad/RUNX2 Signaling Pathway. Biol Trace Elem Res 173: 306-315.
- Mimica-Dukic N, Simin N, Cvejic J, Jovin E, Orcic D, et al. (2008) Phenolic compounds in field horsetail (Equisetum arvense L.) as natural antioxidants. Molecules 13: 1455-1464.
- Stajner D, Popović BM, Canadanović-Brunet J, Boza P (2006) Free radical scavenging activity of three Equisetum species from Fruska gora mountain. Fitoterapia 77: 601-604.
- Rodella LF, Bonazza V, Labanca M, Lonati C, Rezzani R (2014) A review of the effects of dietary silicon intake on bone homeostasis and regeneration. J Nutr Health Aging 18: 820-826.
- Price CT, Koval KJ, Langford JR (2013) Silicon: a review of its potential role in the prevention and treatment of postmenopausal osteoporosis. Int J Endocrinol 2013: 316783.
- Bessa Pereira C, Gomes PS, Costa-Rodrigues J, Almeida Palmas R, Vieira L, et al. (2012) Equisetum arvense hydromethanolic extracts in bone tissue regeneration: in vitro osteoblastic modulation and antibacterial activity. Cell Prolif 45: 386-396.
- Costa-Rodrigues J, Carmo SC, Silva JC, Fernandes MH (2012) Inhibition of human in vitro osteoclastogenesis by Equisetum arvense. Cell Prolif 45: 566-576.
- Macdonald HM, Hardcastle AC, Jugdaohsingh R, Fraser WD, Reid DM, et al. (2012) Dietary silicon interacts with oestrogen to influence bone health: evidence from the Aberdeen Prospective Osteoporosis Screening Study. Bone 50: 681-687.
- Jugdaohsingh R, Tucker KL, Qiao N, Cupples LA, Kiel DP, et al. (2004) Dietary silicon intake is positively associated with bone mineral density in men and premenopausal women of the Framingham Offspring cohort. J Bone Miner Res 19: 297-307.
- Mattioli-Belmonte M, Kyriakidou K, Lucarini G, Gorrieri O, Giavaresi G, et al. (2005) Cell dynamics in the correct control of bone metabolism using natural treatments. Int J Artif Organs 28: 1259-71.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences