ISSN : 2249 - 7412
Asian Journal of Plant Science & Research
The Anti-oxidants and Phytoconstituents of Tiliacora racemosa, Capsicum annum, Trigonella foenum-graecum leaves
1Department of Pharmacy, Gokaraju Rangaraju College of Pharmacy, Osmania University. Telangana State, India
2Department of Pharmacy, KL college of Pharmacy, KL University, Guntur, Andhra Pradesh India
Abstract
Herbs with medicinal properties play an increasingly important role in research to know their functions on disease prevention and treatment property. As the ecosystem is full of potential herbs it becomes very important to study about their beneficial pharmacological responses. This study characterizes the phytochemical composition and antioxidant activity of three medicinal herbs including the leaves of Tiliacora racemosa, Capsicum annum, Trigonella foenum-graecum leaves. These three herbs are well known and familiar for its broad pharmacological responses. It has beneficial responses on all the physiological body systems. By following various chemical methods for phytoconstituents and UV spectrometer for antioxidant studies were carried out using ascorbic acid as standard. The antioxidant studies carried out to determine the potentiality of the herbs selected- Reducing Power Assay and H2O2 radical scavenging assay. Results revealed that the presence of flavonoids, phenols, tannin’s, alkaloids, glycosides, phenols, terpenoids and glycosides despite the well-known antioxidant properties of these plant species, our study suggests all the three herbs can stand out as a relatively more valuable plant source of natural bioactive molecules for developing novel functional property with potential for not only promoting human health against the diseases but also improving bio-valorisation and environment. This study compared and demonstrated these plant extracts as valuable sources of bioactive compounds, likely for preparing novel functional products in various industries for folkloric advantages. These three herbs possess antioxidant properties as well as which can act as scavengers against free radicals, which is one of the causes for various types of diseases.
Methanolic extract of all three herbs is a potential source of natural antioxidants and serves as an effective free radical scavenger and/or inhibitor. Hence, these herbs might be a good plant-based pharmaceutical product for several diseases caused by free radicals, supported by phytoconstituents with presence of abundance amount is surely to be considered for its pharmacological responses.
Keywords
Tiliacora racemosa: Capsicum annum; Trigonella foenum-graecum; Antioxidants; Phytoconstituents; free radicals; Reducing power assay; H2O2 radical scavenging assay
Introduction
There is strong evidence that many dangerous pathophysiological processes, such as cancer, diabetes and cardiovascular and neurodegenerative diseases, are associated with the accumulation of free radicals. A free radical is an atom or molecule that has an unpaired electron and is therefore unstable. This unstable radical has the tendency to become stable through electron pairing with biological macromolecules such as proteins, lipids, and DNA in healthy human cells, thus causing protein and DNA damage [1]. Such radical-caused cell damage can become more widespread due to weakened cellular antioxidant defence systems. All biological systems have innate antioxidant defence mechanisms that remove damaged molecules, but these mechanisms can be inefficient. Therefore, dietary intake of antioxidants is imperative to protect cells from damage caused by free radicals [2,3].
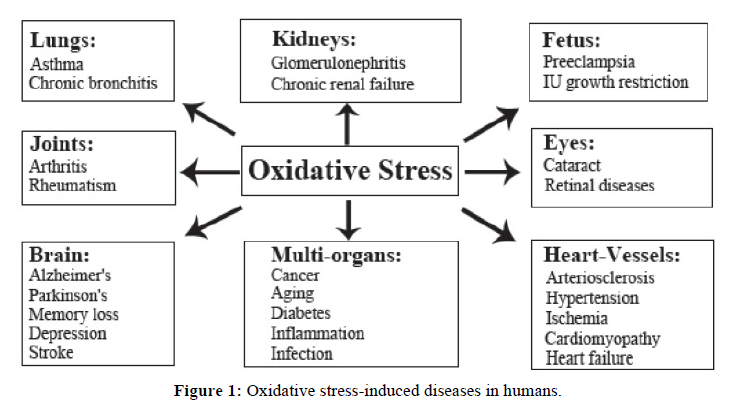
Antioxidants are substances that prevent and stabilize the damage caused by free radicals by supplying electrons from antioxidants to these damage cells. Antioxidants also turn free radicals into waste by-products, which are eliminated from the body. Consumption of antioxidant-enriched fruits and vegetables is known to lower the risk of several diseases caused by free radicals [4-6]. Such health benefits are mainly due to the presence of phytochemicals such as polyphenols, carotenoids, and vitamin E and C. Although phenolic compounds are commonly found in both edible and in non-edible herbs, cereals, fruits, vegetables, oils, spices, and other plant materials, scientific information on the antioxidant properties of endemic plants is scare because the availability of endemic plants is limited to certain regions and only known by local populations [7,8]. Therefore, the assessment of such properties remains an interesting and useful task, particularly to find promising sources of natural antioxidants for functional foods and/or nutraceuticals. Figure 1 [9,10].
Methodology
Preparation of Plant Extract
The freshly collected leaves of the three herbs was collected from local market, cleared from dirt and then the leaves were dried under shade for about 15 days and they were pulverized in the laboratory [11]. Followed by soxhlation to obtain the methanolic extract as the solvent was better suited to obtain all the active constituents needed to study further activities. Authenticated by Dr.P Suresh Kumar, Botanist Govt degree college, Hyderabad T.S voucher number- BSI/ SRC/10/23/2020/Tech/1521, 1023, 304 respectively.
Preliminary phytochemical analysis of the extract -The extract was subjected to preliminary phytochemical investigations to identify various phytoconstituents present in methanolic extract of herbs according to the method prescribed in Khandelwal book [12-14]. These are the following test performed:
a) Tests for Alkaloids:
Evaporate the aqueous, alcoholic and chloroform extracts separately. To residue, add dilute HCl. Shake well and filter. With filtrate, perform the following tests:
•Mayer’s test: 2-3ml, filtrate with few drops Mayer’s reagent gives cream coloured precipitate.
b) Test for Flavonoids
• Shinoda test: To dry powder or extract, add 5ml of 95% Methanol, few drops of concentrated HCl and 0.5g Mg turnings, pink colour observed [15].
• To small quantity of residue, add lead acetate solution. Yellow colour ppt a formed [16,17]. Addition of increasing amount of NaOH to residue, shows yellow coloration, which decolorizes after addition of acid.
c) Test for Phenols
• Ferric chloride test: A fraction of the extracts was treated with aqueous 5% ferric chloride and observed for formation of deep blue or black colour [18].
d) Test for Saponins
• Foam test: To 2 ml of extract was added 6ml of water in a test tube. The mixture was shaken vigorously and observed for the formation of persistent foam that confirms the presence of saponins [19].
e) Test for Steroids
•Liebermann-Burchard test: 2 ml of extract was treated with drops of chloroform, acetic anhydride and conc. Sulphuric acid and observed for the formation of dark pink or red colour [20].
f) Test for Tannins
• Braymer’s test: 2 ml of extract was treated with 10% alcoholic ferric chloride solution and observed for formation of blue or greenish colour solution [21].
Tests for Cardiac Glycosides
g) Test for Terpenoids
• Liebermann’s Burchard’s Test: To 1ml of methanolic extract, add 1ml of chloroform, 2-3ml of acetic anhydride, 1 to 2 drops of concentrated sulphuric acid. Pink to red coloration indicates the presence of terpenoids [22].
• In vitro antioxidant assays
Reducing Power Assay
Principle:
This method is based on the principle of increase in the absorbance of the reaction mixtures. Increase in the absorbance indicates an increase in the antioxidant activity. In this method, substances, which have reduction potential, react with potassium ferricyanide (Fe3+) to form potassium ferrocyanide (Fe2+), which then reacts with ferric chloride to form ferric ferrous complex that has an absorption maximum at 700 nm [23,24].
Potassium ferricyanide Antioxidant Potassium ferrocyanide
Ferric chloride
Ferric ferrous complex (700 nm)
Preparation of reagents:
1. Phosphate buffer pH 6.6: Potassium dihydrogen phosphate (62.5 ml 0.2 M) was added to 250 mL volumetric flask and also 20.5 ml of 0.2 M NaOH and made up to volume 250 ml with distilled water.
2. Potassium dihydrogen phosphate (0.2 M) solution: Potassium dihydrogen phosphate (2.72 g) was dissolved in distilled water and volume made up to 100 ml [25].
3. Sodium hydroxide solution (0.2 M) solution: 0.8 g of sodium hydroxide was dissolved in distilled water and volume made up to 100 ml [26].
4. Potassium ferricyanide (1% w/v) solution: Potassium ferricyanide (1 g) was dissolved in water and volume made up to 100 mL in volumetric flask.
5. Ferric chloride solution (0.1% w/v): Ferric chloride (25 mg) was dissolved in distilled water and volume made up to 25 mL in volumetric flask.
Method:
1. To 1 mL of test and standard compounds added 2.5 mL of potassium ferricyanide (1 % w/v), 2.5 mL of phosphate buffer pH 6.6 and incubated at 500C for 30 min.
2. To 2.5 mL of above supernatant liquid added 2.5 mL of distilled water and 0.5 mL of FeCl3 solution (0.1% w/v).
3. The absorbance of ferric ferrous complex was measured using phosphate buffer pH 6.6 as control at 700 nm using UV-Visible spectrophotometer and estimated the increase in absorbance.
4. The percent increase in reducing power was calculated using the following equation.

Where ‘Abstest’ is absorbance of test solution
‘Absblank’ is absorbance of blank.
H2O2 Radical Scavenging Assay
Principle- Hydrogen peroxide is a weak oxidizing agent and can inactivate a few enzymes directly, usually by oxidation of essential thiol (-SH) groups. Hydrogen peroxide can cross cell membranes rapidly, once inside the cell, H2O2 can probably react with Fe2+, and possibly Cu2+ ions to form hydroxyl radical and this may be the origin of many of its toxic effects. It is therefore biologically advantageous for cells to control the amount of hydrogen peroxide that is allowed to accumulate.
Preparation of reagents:
1. Phosphate buffer solution pH 7.4: Add 250.0 mL of 0.2 M potassium dihydrogen phosphate to 393.4 mL of 0.1 M sodium hydroxide and make up the volume to 1000 mL with the distilled wáter [27,28].
2. Potassium dihydrogen phosphate (0.2 M) solution: Potassium dihydrogen phosphate (2.72 g) was dissolved in distilled water and volume made up to 100 ml.
3. Sodium hydroxide solution (0.1 M) solution: 0.4 g of sodium hydroxide was dissolved in distilled water and volume made up to 100 ml.
Method
1. A solution of hydrogen peroxide (2 mmol/l) was prepared in phosphate buffer (Ph 7.4).
2. Test compounds (10–50 μg/M) were added to hydrogen peroxide solution (0.6 mL).
3. Absorbance of hydrogen peroxide at 230 nm was determined after 10 min against a blank solution containing phosphate buffer without hydrogen peroxide and compared with ascorbic acid, the reference compound.
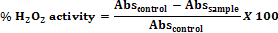
Where, Abs (control): Absorbance of the control
Abs (sample): Absorbance of the extract/standard.
In vitro Antioxidant Assays
The methanolic extract of all the three herbs was subjected to in vitro antioxidant activity. In vitro anti-oxidant activity was performed using reducing power assay & hydrogenperoxide scavenging assay.
Result
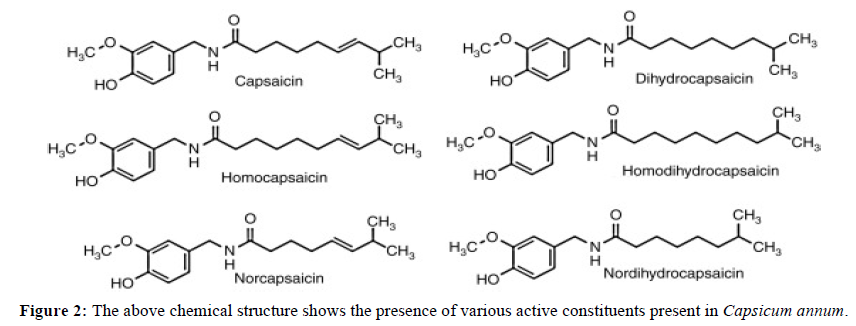
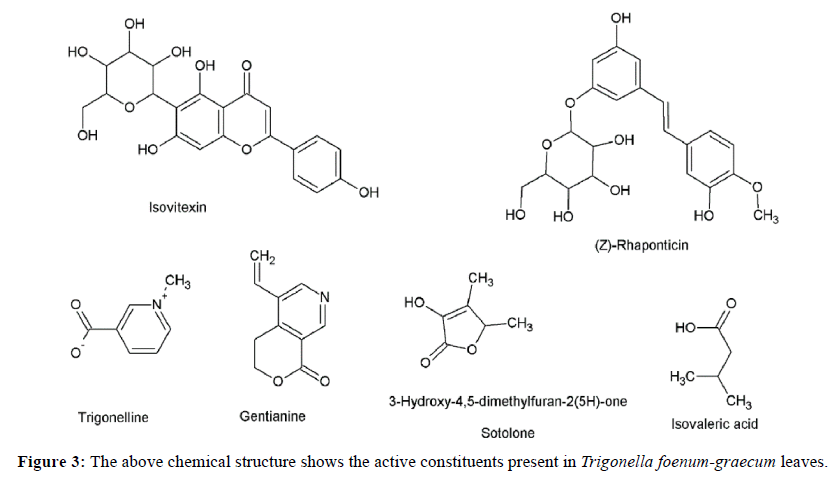
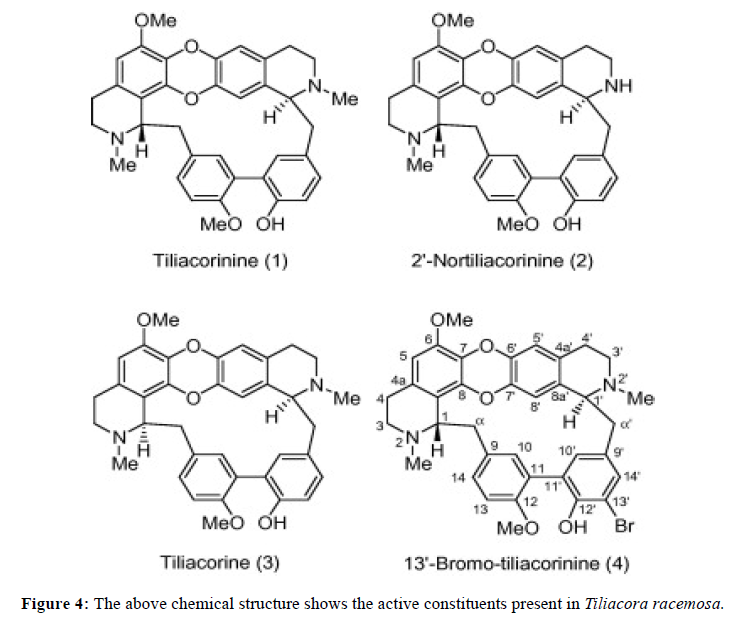
Following is the result of phytoconstituents present in three different herbs using methanol extract by following chemical methods to determine it Figure 2,3,4,5 Table 1.
| S.no | Name of Phytochemical Constituents |
Results Analysis of Methanolic Extract of Tiliacora Racemosa |
Results Analysis of Methanolic Extract of Trigonella Foenum Graecum |
Results Analysis of Methanolic Extract of Capsicum annum |
|---|---|---|---|---|
| 1. | Alkaloids | + | + | + |
| 2. | Phenols | + | + | + |
| 3. | Saponins | + | + | + |
| 4. | Tannins | + | + | + |
| 5. | Flavonoids | + | + | + |
| 6. | Anthraquinones | + | + | + |
| 7. | Glycosides | + | + | + |
| 8. | Terpenoids | + | + | + |
| 9. | Steroids | - | - | - |
Table 1: By the evidence of above constituents, it can be selected to perform various activities on physiological system disorders
Anti-oxidant studies- The methanolic extract of all the three has shown antioxidant activity in reducing power assay. The reducing ability of a compound generally depends on the presence of reductants which have been exhibiting antioxidative potential by breaking the free radical chain and donating a hydrogen atom.
The reducing capacity of a compound can be known by measuring Fe3+- Fe2+ complex. Fe3+ reduction is due to electron donating activity of herbs, which is an important mechanism of antioxidant action. Increase in absorbance indicates an increase in reductive ability of hers. The reducing power activity of herbs might be due to presence of hydroxyl groups. It might be due to the presence of phenols and flavonoids the extract with adequate number of hydroxyl groups [29,30].
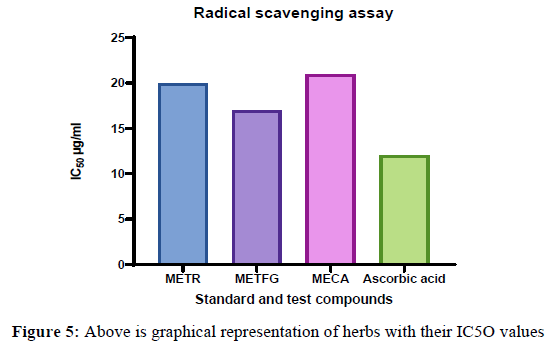
Tiliacora racemose, Trogenella feonum graceum and capsicum annum had shown dose dependent inhibition of free radicals and its IC50 value was found to be 32, 28, 34 μg/mL respectively.The potential of the extract was comparable to that of standard ascorbic acid (IC50 = 26 μg/mL). From the above result it is confirmed that Trogenella feonum graceum is more potent than other two herbs, because of its IC50 which is very close to ascorbic acid.
Hydrogen Peroxide Scavenging Assay
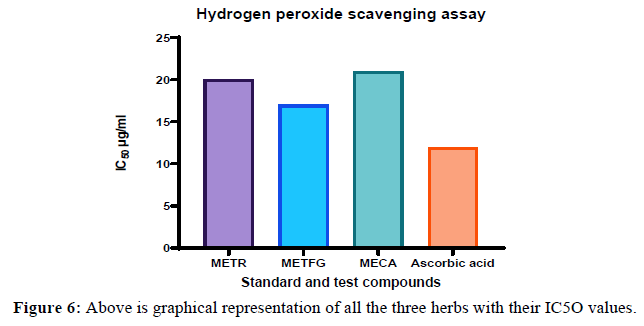
The in vitro antioxidant activity was performed using hydrogen peroxide scavenging assay. The IC50 value of the Tiliacora racemose, Trogenella feonum graceum and capsicum annum was 20, 17, 21 μg/mL & ascorbic acid was 12 μg/mL respectively. From the results it is clear that three herbs showed antioxidant activity Table 2,3,4,5,6,7.
| S.no | Compounds | Concentration | % Inhibition | IC50 Values |
|---|---|---|---|---|
| 1. | Ascorbic acid | 10 20 30 40 50 |
31.62 ± 0.40 45.07 ± 0.25 56.67 ± 0.25 64.36 ± 0.10 70.08 ± 0.77 |
26 |
| 2. | METR | 10 20 30 40 50 |
22.08 ± 0.52 35.57 ± 0.35 49.49 ± 0.33 61.26 ± 0.12 65.22 ± 0.15 |
32 |
Table 2: Reducing power assay of methanolic leaf extract of Tiliacora racemosa.
| S.no | Compounds | Concentrations | % Inhibition | IC50 Values |
|---|---|---|---|---|
| 1. | Ascorbic acid | 10 20 30 40 50 |
48.62 + 0.41 69.35 + 0.14 79.26 + 0.03 83.78 + 0.01 86.04 + 0.06 |
12 |
| 2. | METR | 10 20 30 40 50 |
43.27 + 0.48 53.64 + 0.32 73.23 + 0.10 80.41 + 0.05 84.46 + 0.02 |
20 |
Table 3: Hydrogen peroxide scavenging assay of methanolic leaf extract of Tiliacora racemosa
| S.NO | Compounds | Concentrations | % Inhibition | IC50 Values |
|---|---|---|---|---|
| 1. | Ascorbic acid | 10 20 30 40 50 |
31.62 ± 0.40 45.07 ± 0.25 56.67 ± 0.25 64.36 ± 0.10 70.08 ± 0.77 |
26 |
| 2. | METGF | 10 20 30 40 50 |
25.68 ± 0.22 35.87 ± 0.45 51.59 ± 0.34 58.29 ± 0.19 62.27 ± 0.18 |
28 |
Table 4: Reducing power assay of methanolic leaf extract of Trigoella feonum graceum [METFG].
| S.NO | Compounds | Concentrations | % Inhibition | IC50 Values |
|---|---|---|---|---|
| 1 | Ascorbic acid | 10 20 30 40 50 |
48.62 + 0.41 69.35 + 0.14 79.26 + 0.03 83.78 + 0.01 86.04 + 0.06 |
12 |
| 2 | METGF | 10 20 30 40 50 |
39.08 ± 0.43 46.57 ± 0.56 49.79 ± 0.83 59.16 ± 0.73 63.12 ± 0.65 |
17 |
Table 5: Hydrogen peroxide scavenging assay of methanolic extract of trigonella feonum graceum [METGF]
| S.NO | Compounds | Concentrations | % Inhibition | IC50 Values |
|---|---|---|---|---|
| 1 | Ascorbic acid | 10 20 30 40 50 |
31.62 ± 0.40 45.07 ± 0.25 56.67 ± 0.25 64.36 ± 0.10 70.08 ± 0.77 |
26 |
| 2 | MECA | 10 20 30 40 50 |
18.09 ± 0.51 27.51 ± 0.23 39.46 ± 0.54 49.24 ± 0.87 61.21 ± 0.71 |
34 |
Table 6: Reducing power assay of methanolic leaf extract of Capsicum annum/racemosa [MECA].
| S.NO | Compounds | Concentrations | % Inhibition | IC50 Values |
|---|---|---|---|---|
| 1 | Ascorbic acid | 10 20 30 40 50 |
48.62 + 0.41 69.35 + 0.14 79.26 + 0.03 83.78 + 0.01 86.04 + 0.06 |
12 |
| 2 | MECA | 10 20 30 40 50 |
31.08 ± 0.23 42.57 ± 0.54 55.49 ± 0.56 67.26 ± 0.78 71.22 ± 0.49 |
21 |
Table 7: Hydrogen peroxide scavenging assay of methanolic leaf extract of Capsicum annum/racemosa.
Statistical analysis The percentage inhibition was calculated using: Percent Inhibition = Ac-As/ Ac x 100
Where, Ac is absorbance of control,
As is the absorbance of sample.
IC50 value (a concentration at 50% inhibition) was determined from the curve between percentage inhibition and concentration. All determinations were done in triplicate and the IC50 value was calculated by using the equation of line (Figure 6) [31-34].
Discussion
The body has several mechanisms to counteract oxidative stress by producing antioxidants, either naturally generated in situ (endogenous antioxidants), or externally supplied through foods (exogenous antioxidants). The roles of antioxidants are to neutralize the excess of free radicals, to protect the cells against their toxic effects and to contribute to disease prevention. The implication of oxidative stress in the etiology of several chronic and degenerative diseases suggests that antioxidant therapy represents a promising avenu treatment [35-37]. In the future, a therapeutic strategy to increase the antioxidant capacity of cells may be used to fortify the long-term effective treatment. However, many questions about antioxidant supplements in disease prevention remain unsolved. Further research is needed before this supplementation could be officially recommended as an adjuvant therapy. In the meantime, it is reminded that avoiding oxidant sources (cigarette, alcohol, bad food, stress, etc) must be considered as important as taking diet rich in antioxidants. Indeed, our health also depends on our lifestyle.
Conclusion
Due to the presence of various types of phytoconstituents all the three herbs possess to have properties of curing various kinds of diseases. The two methods used to study anti-oxidant properties provide the evidence of having rich inhibiting free radical properties. Further investigations and intensive research on these herbs will provide sufficient knowledge of their constituents and anti-oxidant properties which is going to be helpful in many diseases
References
- Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D. Antioxidant therapy in acute central nervous system injury: current state. Pharmacol Rev. 2002, 54:271–84.
- Hamid K, Saha MR, Urmi KF, Habib MR, Rahman MM, et al. Screening of different parts of the plant Pandanus odorus for its antioxidant activity. Int J Appl Biol Pharm. 2010, 1:1364–8.
- Balasundram N, Sundram K, Samman S. Phenolic compounds in plants and agri-industrial by products: antioxidant activity, occurrence, and potential uses. Food Chem. 2006; 99:191–203.
- Ramalakshmi S, Muthuchelian K. Analysis of bioactive constituents from the ethanolic leaf extract of Tabebuia rosea (Bertol.) DC by gas chromatography-mass spectrometry. Int J Chem Tech Res. 2011, 3:1054–9.
- Wolfe K, Wu X, Liu RH. Antioxidant activity of apple peels. J Agric Food Chem. 2003 ,51:609–14.
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958, 181:1199–200.
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. Clarendon Press. 1989, 3:617–783
- Kedare BS, Singh RP. Genesis and development of DPPH method of antioxidant assay. J Food Sci Technol. 2011, 48:412–22.
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 4th. Oxford, UK: Clarendon Press; 2007.
- Bahorun T, Soobrattee MA, Luximon-Ramma V, Aruoma OI. Free radicals and antioxidants in cardiovascular health and disease. Internet J Med. Update. 2006, 1:1–17.
- Valko M, Izakovic M, Mazur M, Rhodes CJ. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004, 266:37–56.
- Valko M, Leibfritz D, Moncola J, Cronin MD. Free radicals and antioxidants in normal physiological functions and human disease. Review. Int J Biochem Cell Biol. 2007, 39:44–84.
- Droge W. Free radicals in the physiological control of cell function. Review Physiol Rev. 2002, 82:47–95.
- 6. Willcox JK, Ash SL, Catignani GL. Antioxidants and prevention of chronic disease. Review. Crit. Rev. Food. Sci. Nutr. 2004, 44:275–295.
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87:315–424.
- Genestra M. Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Review Cell Signal. 2007, 19:1807–1819.
- Halliwell B. Biochemistry of oxidative stress. Biochem Soc Trans. 2007, 35:1147–1150.
- Young I, Woodside J. Antioxidants in health and disease. J Clin Pathol. 2001, 54:176–186.
- Dabanka CP. Antibacterial activity of phyllanthusamarus (schumand thonn) extract against salmonella typhicausative agent of typhoid fever. 2013.
- B. Sanyal and A. Sharma, “Cytological studies in Indian Cyperaceae,” Cytologia, vol. 37, no. 1, pp. 13–32, 1972.View at: Publisher Site | Google Scholar
- Qasim M, Gulzar S, Khan MA. Halophytes as medicinal plants,” in Proceedings of the NAM Meeting. 2011.
- Abrosca BD, Dellagreca M, Fiorentino A, Isidori M, Monaco P, et al. Chemical constituents of the aquatic plant Schoenoplectus lacustris: evaluation of phytotoxic effects on the green alga Selenastrum capricornutum. Journal of Chemical Ecology. 2006, 32(1):81–96,
- Wu CG, Tseng HY, Chen ZC. Fungi inhabiting on Schoenpplectus triqueter (L.) palla (I). 1982. 27(1):35-38,
- Batool B, Hameed M. Root structural modifications in three Schoenoplectus (Reichenb.) Palla species for salt tolerance. Pakistan Journal of Botany. 2013. 45(16):1969–1974.
- Fay M, Cowan R, Simpson D. Hybridisation between Schoenoplectus tabernaemontani and S. Triqueter (Cyperaceae) in the British isles. 2003, 24(3):433–442.
- Jiménez-Mejías P, Luceno M, astroviejo S. Schoenoplectus corymbosus: a tropical old-world sedge (Cyperaceae) discovered in Spain and Morocco. Nordic Journal of Botany. 2007, 25:70–74.
- Roalson EH. A synopsis of chromosome number variation in the Cyperaceae. The Botanical Review. 2008, 74(2):209-393.
- Hertog MGL, Feskens EJM, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly study. The Lancet. 1993. 342(8878):1007–1011.
- Moure A, Cruz JM, Franco D. Natural antioxidants from residual sources. Food Chemistry. 2001. 72(2):145–171.
- Holiman PCH, Hertog MGL, Katan MB. Analysis and health effects of flavonoids. Food Chemistry. 1996, 57(1):43–46.
- Meyer AS, Heinonen M, Frankel EN. Antioxidant interactions of catechin, cyanidin, caffeic acid, quercetin, and ellagic acid on human LDL oxidation. Food Chemistry. 1998. 61(1):71–75.
- Hunt EJ, Lester CE, Lester A, Tackett RL. Effect of St. John’s wort on free radical production. Life Sciences. 2001, 69(2):181–190.
- Kibwage IO, Mwangi J, Thoithi G. Quality control of herbal medicines. East and Central African Journal of Pharmaceutical Sciences. 2005, 8(2):27–30.
- Hammer KA, Carson CF, Riley TV. Antimicrobial activity of essential oils and other plant extracts. Journal of Applied Microbiology. 1999, 86(6):985–990.
- Suffredini BI, Sader HS, Gonçalves AG. Screening of antibacterial extracts from plants native to the Brazilian amazon rain forest and atlantic forest. Brazilian Journal of Medical and Biological Research. 2004, 37(3):379–384.
- Hassan A, Ullah H. Antibacterial and antifungal activities of the medicinal plant veronica biloba. Journal of Chemistry. 2019.
- Hetty Manurung RA, Nugroho RA, Puspita Sari Y, Chernovita R, Auliana et al. Phytochemical analysis and antioxidant activity of leaves extracts of endemic plant jahe balikpapan (etlingera balikpapanensis A.D. Poulsen). International Journal of Scientific & Technology Research. 2019. 8(9).

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences