ISSN : 0976-8505
Der Chemica Sinica
Synthetic Methodology of Manganese Corroles: Recent Developments
Department of Applied Chemistry, Delhi Technological University, Bawana Road, Delhi 42, India
Abstract
Let Un be the set of unicyclic molecular graphs with 3 ≤ n ≤ 8 vertices. We show that the cycle Cn has maximal Laplacian-energy-like invariant (LEL) in Un. The authors partially proving that the conjecture hold for any unicyclic molecular graph in Un, where 3 ≤ n ≤ 8 Moreover, we show that Cn has maximal energy (E) in Un for 3 ≤ n ≤ 7, but for n=8 this is not true.
Keywords:
Molecular graphs, Laplacian energy-like invariant, Energy
Introduction
Corrole is trianionic tetrapyrrolic macrocycle containing an eighteen pi-electron having a direct link between both pyrrole–pyrrole units [1,2]. The structure of corrolazine, corrine and porphyrine is in close resemble to corrole. Porphyrin has a larger cavity and higher symmetry than corrole due to direct pyrrole-pyrrole link. Therefore corrole has C2v symmetry [3]. The most interesting properties of the corroles is the trianionic nature due to this properties corrrole stabiles metal ions in higher oxidation (+4 to +6) state [4-7]. These ultimate properties are responsible for the huge utility of manganese and iron corroles for great important aspects compatible to medicine and energy , because they initiate the molecules as like: O2 [8], H2O2 [9], HOONO [10],CO2, [11], H+[12] and more. Metallocorroles already reported as catalysts for many applications such as transfer of oxygen atom, CO2 and reduction of protons [12-22]. Application of manganese corroles reported in catalysis [23-27], ion-selective electrodes [28], Langmuir– blodgett films [29,30], single-chain magnets [31] and medicinal research [32,33]. Gross et al. reported the synthesis of tris(pentaflurophenyle)corrole in good yield using solvent free metthod [28-30]. Furthermore, the synthesis of corrole macrocycle reported by Kumar [31] and Paolesse et al. [32] by condensation of dipyrromethane (DPM) with aromatic aldehyde, and by the Rothemund reaction respectively. This synthetic achievement has expedited the study of macrocycle of the corrole a new branch of porphyrin chemistry. It has been clearly seen that in area of corrole chemistry increased exponentially a huge publication till now [33-44]. This review is mainly accommodates all synthetic aspect in manganese corrole chemistry from 1999 to 2018.
Results and Discussion
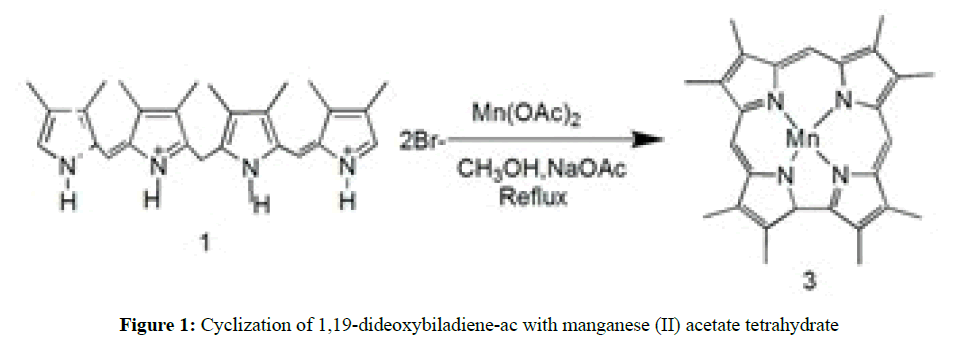
Recently, few general approaches for the synthesis of manganese corroles were reported. First, use of manganese salt to cyclize tetrapyrrolic precursor and the second, is the reaction of manganese salt with free base corrole. The third report recently reported by Yadav et al. by using manganese salt with bilane to yield metallocorroles through difficult C-C bond formations. In the first methodology, cyclization of 1,19-dideoxybiladiene-ac in methanol and basic condition using manganese(II) acetate tetrahydrate as template to yield octamethylcorrolato manganese(III)s in good yield 50%-60% [45,46] has been reported (Figure 1).
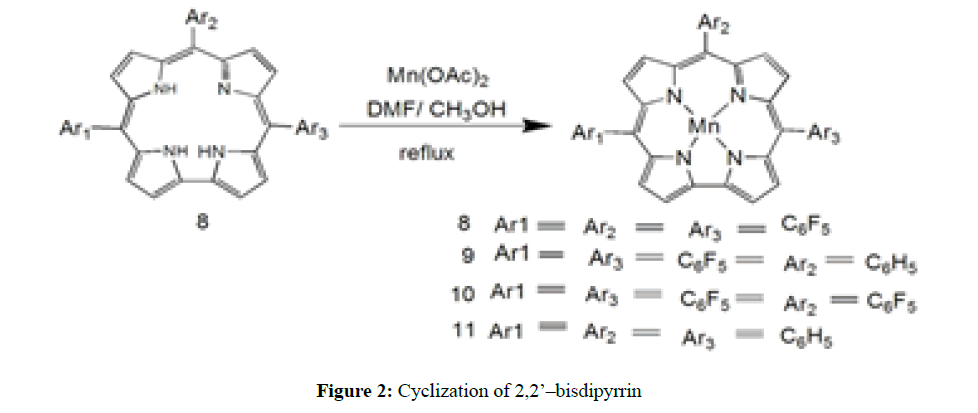
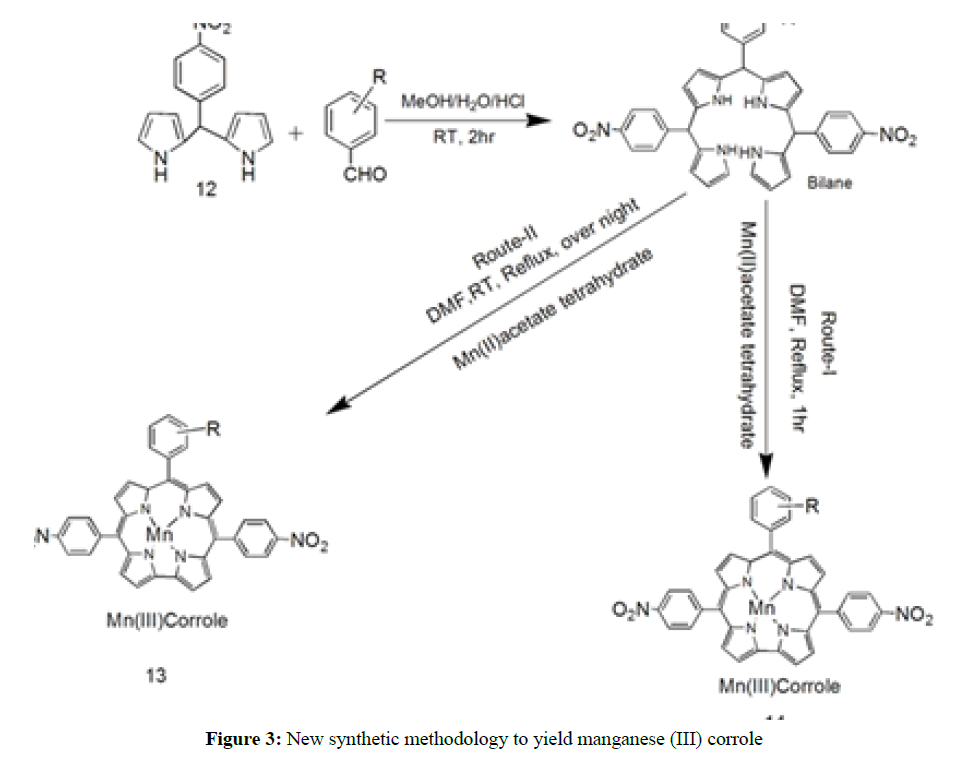
In second methodology, as shown in Figure 2, using two equivalent of Mn(II) acetate and molecular oxygen in boiling DMF to cyclize tetrapyrrolic precursor of 2,2’/-Bisdipyrrin to give 18% yield of octaethylcorrolatomanganese(III). In the series of facile synthetic protocol corroles, an another alternative most convenient method is reaction between free base corrole and manganese(II) acetate tetrahydrate to obtain Mn(III) corrole [8-11] in 90%-98% yield in presence of polar solvent such as dimethylformaamide, methanol or pyridine [45-50]. Corroles have better solubility in polar solvents in comparison to other porphyrinoid systems. Ionic corroles form metal complexes easily with manganese by the same method [51-55]. And in the last not but least recently Yadav et al. reported compound 12 to 14, new C-C coupling between both pyrroles in bilane using manganese(II) acetate tetrahydrate as template in the presence of molecular oxygen in DMF to obtain 30%-35% yield of manganese (III) corrole as shown in Figure 3.
Demetallation of manganese corroles
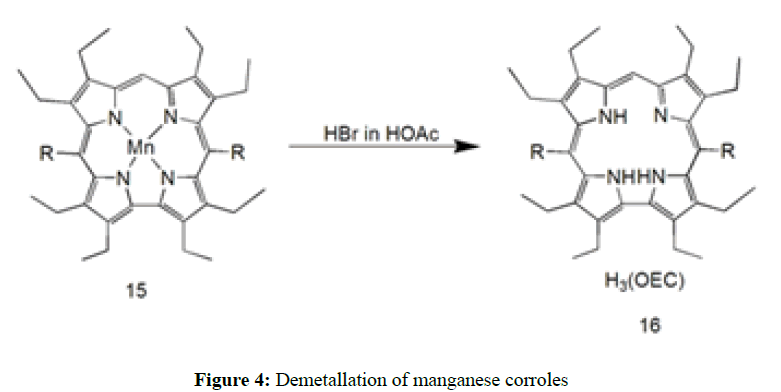
The synthesis of highly electron-deficient and perhalogenated free base corrole can be achieved with the reductive demetallation of manganese corroles but did not gain much attention. Bröring et al. firstly reported by using HBr in HOAc (Figure 4) for demetallation of (OEC)Mn(III) 15 to yield H3(OEC) [56]. Another method was reported by Bröring et al. [57] by using acid mixture of 10:1 volume ratio of CHCl3/H2SO4. Demetallation of beta-octabromomeso- triarylcorroles are possible by using concentrated H2SO4 with 5-200 equivalent of FeCl2 or SnCl2 H3(Br8tpfc), in generally through reductive demetallation of (Br8tpfc)Mn(III). We can be obtained in 86% yield using concentrated H2SO4 in the presence of FeCl2 [57]. Without reductant demetallation also reported by Liu et al. in presence of HCl with SnCl2 as well as in HOAc-H2SO4.
Conclusion
This short review provides the all synthetic methodology and demetallation process of manganese(III)corrole from 1999 to 2018. Furthermore, the main purpose of this review to provide information about currently reported synthetic methodology in manganese(III) corroles. The prestige of this protocol is the very simple reaction which is applicable for all type of aromatic aldehyde and aryldipyrromethane. This method also avoids the difficult steps of earlier reported by Broring et al. in compound 2 to 6. Mainly green chemistry protocol has been adopted in this methodology. The yield of the synthesized metallocorroles was plausible high and also need very short time period than earlier reported methods.
References
- Paolesse R, in: Kadish KM, Smith KM, Guilard (Eds.) R (2000) The Porphyrin Handbook,vol. 2, Academic, San Diego, U.S.A.
- Erben C, Will CS, Kadish KM (2000) The Porphyrin Handbook,vol. 2, Academic, San Diego, U.S.A.
- Adamian VM,Souza FD, Licoccia S, Di Vona ML, Tassoni E (1995) Synthesis, characterization, and electrochemical behavior of (5,10,15-Tri-X-phenyl-2,3,7,8,12,13,17,18-octamethylcorrolato)cobalt(III) triphenylphosphine complexes, where X = p-OCH3, p-CH3, p-Cl, m-Cl, o-Cl, m-F, or o-F. Inorg Chem 34: 532-540.
- Gross Z, Galili N, Saltsman I (1999) The first direct synthesis of Corroles from Pyrrole. Angew Chem Int Ed 38: 1427-1429.
- Gross Z, Galili N, Simkhovich L, Saltsman I, Botoshansky M, et al. (1999) Solvent-free condensation of pyrrole and pentafluorobenzaldehyde: A novel synthetic pathway to corrole and oligopyrromethenes. Org Lett 1: 599-602.
- Gryko DT, Katarzyna J (2000) A simple, rational synthesis of meso-Substituted A2B-corroles. Chem Commun 32: 2243-2244.
- Paolesse R, Nardis S, Sagone F, Khoury RGJ (2001) Synthesis and Functionalization of meso-Aryl-Substituted Corroles. J Org Chem 66: 550-556.
- Gross Z, Golubkov G, Simkhovich L (2000) Epoxidation catalysis by a manganese corrole and isolation of an oxomanganese (V) corrole. Angew Chem Int Ed 39: 4045-4047.
- Golubkov G, Bendix J, Gray HB, Mahammed A, Goldberg I, et al. (2001) High-valent manganese corroles and the first perhalogenated metallocorrole catalyst. Angew Chem Int Ed 40: 2132-2134.
- Paolesse R, Pandey RK, Forsyth TP, Jaquinod L, Gerzevske KR, et al. (1996) Stepwise syntheses of bisporphyrins, bischlorins, and biscorroles, and of porphyrin−chlorin and porphyrin−corrole heterodimers. J Am Chem Soc 118: 3869-3882.
- Paolesse R, Sagone F, Macagnano A, Boschi T, Prodi L, et al. (1999) Photophysical behaviour of corrole and its symmetrical and unsymmetrical dyads. J Porphyrins Phthalocyanines 3: 364-370.
- Gryko DT (2002) Recent advances in the synthesis of corroles and core‐modified corroles. Eur J Org Chem 1735-1743.
- Ghosh (2004) A perspective of one‐pot pyrrole–aldehyde condensations as versatile self‐assembly processes. Angew Chem Int Ed 43: 1918-1931.
- Koszarna B, Gryko DT (2006) Efficient synthesis of meso-substituted corroles in a H2O−MeOH mixture. J Orga Chem 71: 3707-3717.
- Sessler JL, Weghorn SJ (1998) Expanded, contracted and isomeric porphyrins. pergamon press: Oxford, U.K.
- Palmer JH, Durrell AC, Gross Z, Winkler JR, Gray HB (2010) Near-IR phosphorescence of iridium(III) corroles at ambient temperature. J Am Chem Soc 132: 9230-9231.
- Kadish km, Frémond l, Shen J, Chen P, Ohkubo K, et al. (2009) Catalytic activity of biscobalt porphyrin-corrole dyads toward the reduction of dioxygen. Inorg Chem 48: 2571-2582.
- Liu S, Mase K, Bougher C, Hicks SD, Abu-Omar MM, et al. (2014) High-valent chromium–oxo complex acting as an efficient catalyst precursor for selective two-electron reduction of dioxygen by a ferrocene derivative. Inorg Chem 53: 7780-7788.
- Aviv-Harel I, Gross Z (2009) Aura of corroles. Chem Eur J 15: 8382-8394.
- Abu-Omar MM (2011) High-valent iron and manganese complexes of corrole and porphyrin in atom transfer and dioxygen evolving . Dalton Trans 40: 3435-3444.
- Mahammed A, Gray HB, Meier-Callahan AE, Gross Z (2003) Aerobic oxidations catalyzed by chromium corroles. J Am Chem Soc. 125: 1162-1163.
- Collman JP, Kaplum M, Decréau RA (2005) Metal corroles as electrocatalysts for oxygen reduction. Dalton Trans, 2006: 554-559.
- Gryko DT, Jadach K (2001) A simple and versatile one-pot synthesis of meso-Substituted trans-A2B-corroles. J Org Chem 66: 4267-4275.
- Gryko DT, Piechota KE (2002) Straightforward route to trans-A2B-corroles bearing substituents with basic nitrogen atoms. J Porphyrins Phthalocyanines 6: 81-97.
- Gross Z, Mahammed A (2002) Selective sulfonation and deuteration of free-base corroles. J Porphyrins Phthalocyanines 6: 553-555.
- Gryko DT, Tasior M, Koszarna B (2003) parallel synthesis of meso-substituted corroles and meso-substituted [22]pentaphyrins(1.1.1.0.0) from diacyl-dipyrromethanes. J Porphyrins Phthalocyanines 7: 239-248.
- Yadav O, Varshney A, Kumar A, Ratnesh RK , Mehata MS (2018) A2B corroles: Fluorescence signaling systems for sensing fluoride ions. Spectrochim Acta A Mol Biomol Spectrosc 202: 207-213.
- Rohand T, Dolusic E, Ngo TH, Maes W, Dehaen (2007) Efficient synthesis of aryldipyrromethanes in water and their application in the synthesis of corroles and dipyrromethenes. ARKIVOC 307-314.
- Decréau RA, Collman JP (2003) Corrole synthesis by dipyrromethane- dicarbibinol and 2,2'- bipyrrole condensation. Tetrahedron Lett 44: 3323-2327.
- Golubkov G, Gross Z (2005) Nitrogen atom transfer between manganese complexes of salen, porphyrin, and corrole and characterization of a (nitrido) manganese (VI) corrole. J Am Chem Soc 127: 3258-3259.
- Kumar A, Goldberg I, Botoshansky M, Buchman Y, Gross Z (2010) Oxygen atom transfer reactions from isolated (Oxo)manganese(V) corroles to sulfides. J Am Chem Soc 132: 15233-15245.
- Paolesse R, Mini S, Sagone F, Boschi T, Jaquinod L, et al. (1999) 5,10,15-Triphenylcorrole: A product from a modified Rothemund reaction. Chem Commun 1999: 1307-1308.
- Barbe JM, Canard G, Brandes S, Guilard R (2005) Synthesis and physicochemical characterization of meso‐functionalized corroles: Precursors of organic–inorganic hybrid. Eur J Org Chem 2005: 4601-4611.
- Hiroto S, Hisaki I, Shinokubo H, Osuka A (2005) Synthesis of corrole derivatives through regioselective ir‐catalyzed direct borylation. Angew Chem Int Ed 44: 6763-6766.
- Gryko DT (2008) Adventures in the synthesis of meso-substituted corroles. J Porphyrins Phthalocyanines 12: 906-917.
- Koszarna B, Gryko DT (2006) One-pot synthesis of meso-tris-aryl-substituted N-21-methyl- and N-21-benzyl-corroles. Tetrahedron Lett. 47: 6205-6207.
- Maes W, Ngo TH, Vanderhaeghen J, Dehaen W (2007) meso-pyrimidinyl-substituted A2B-corroles. Org Lett 9: 3165-3168.
- Gryko DT, Piechowska J, JaworskiM JS, Galezowski M, Tasior M, et al. (2007) Synthesis and properties of directly linked corrole–ferrocene systems. New J Chem 31: 1613-1619.
- Paolesse R, Licoccia S, Fanciullo M, Morgante E, Boschi T (1993) Synthesis and characterization of cobalt(III) complexes of meso-phenyl-substituted corroles. Inorg Chim Acta 203: 107-114.
- Palmer JH, Mahammed A, Lancaster KM, Gross Z, Gray HB (2009) Structures and reactivity patterns of group 9 metallocorroles. Inorg Chem 48: 9308-9315.
- Zhan HY, Liu HY, Chen HJ, Jiang HF (2009) Preparation of meso-substituted trans-A2B-corroles in ionic liquids. Terahedron Lett 50: 2196-2199.
- Shi L, Liu HY, Si LP, Peng KM, You LL et al. (2010) The heavy atom effect on photocleavage of DNA by mono-hydroxyl halogenated corroles. Chin Chem Lett 21: 373-375.
- Shi L, Liu HY, Peng KM, Wang XL, You LL, et al. (2010) Synthesis of phenothiazine-corrole dyads: The enhanced DNA photocleavage properties. Terahedron Lett 51: 3439-3442.
- Dong SS, Nielsen RJ, Palmer JH, Gray HB, Gross Z, et al. (2011) Electronic structures of group 9 metallocorroles with axial ammines. Inorg Chem 50: 764-770.
- Gross Z, Golubkov G, Simkhovich L (2000) Epoxidation catalysis by a manganese corrole and isolation of an oxomanganese(V) corrole. Angew Chem Int Ed 39: 4045-4047.
- Golubkov G, Bendix J, Gray HB, Mahammed A, Goldberg I, et al. (2001) High-valent manganese corroles and the first perhalogenated metallocorrole. Angew Chem Int Ed. 40: 2132-2134.
- Liu HY, Lai TS, Yeung LL, Chang CK (2003) First synthesis of perfluorinated corrole and its Mn=O complex. Org Lett 5: 617-620.
- Gross Z, Gray HB (2004) Oxidations catalyzed by metallocorroles. Adv Synth Catal 346: 165-170.
- Lvova L, Natale CD, Amico AD, Paolesse R (2009) Corrole-based ion-selective electrodes. J Porphyrins Phthalocyanines 13: 1168-1178.
- Paolesse R, Natale CD, Macagnano A, Sagone F, Scarselli MA, et al. (1999) Langmuir-blodgett films of a manganese corrole derivative. Langmuir 15: 1268-1274.
- Ding M, Wang B, Wang Z, Zhang J, Fuhr O, et al. (2012) Constructing single‐chain magnets by supramolecular π–π stacking and spin canting: A case study on manganese (III) corroles. Chem Eur J 18: 915-924.
- Okun Z, Kupershmidt L, Youdim MBH, Gross Z (2011) Cellular uptake and organ accumulation of amphipolar metallocorroles with cytoprotective and cytotoxic properties. Anticancer Agents Med Chem 11: 380-384.
- Lim P, Mahammed A, Okun Z, Saltsman I, Gross Z, et al. (2012) Differential cytostatic and cytotoxic action of metallocorroles against human cancer cells: Potential platforms for anticancer drug development. Chem Res Toxicol 25: 400-4009.
- Mc Gown AJ, Badiei YM, Leeladee P, Prokop KA, DeBeer S, et al. (2011) The porphyrin handbook, world scientific press. San Diego, U.S.A.
- Saltsman I, Mahammed A, Goldberg I, Tkachenko E, Botoshansky M, et al. (2002) Selective substitution of corroles: Nitration, hydroformylation, and chlorosulfonation. J Am Chem Soc 124: 7411-7420.
- Yadav O, Varshney A, Kumar A (2017) Manganese(III) mediated synthesis of A2B Mn(III) corroles: A new general and green synthetic approach and characterization. Inorg Chem Commun 86: 168–171.
- Bröring M, Hell C (2001) Manganese as a template: a new synthesis of corrole. Chem Commun 2336-2337.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences