ISSN : 0976-8505
Der Chemica Sinica
Synthesis, Spectral Characterization, Electrochemical Behaviour, In vitro Antimicrobial and DPPH Radical Scavenging Activities of Iron (II), Cobalt (II) Complexes with Imidazolyl Terpyridine
G. Krishnaveni1*, M Seeni Mubarak2, M Kiruthika3 and R Elayaperumal1
1Assistant Professor, J.J. College of Engineering and Technology, Tiruchirappali-620009, India
2Associate Professor, Jamal Mohamed College, Tiruchirappalli-620020, India
3Assistant Professor, Arinar Anna Government Arts College, Musiri, India
Abstract
Imidazole terpyridine (L) and its Fe(II), Co(II) metal complexes were synthesized. IR, UV-vis, Mass, 1HNMR, elemental analysis, electrical conductance and magnetic moments is techniques were applied for characterization. Cyclic voltammetry studies of Fe(II) and Co(II) complexes in DMSO show that the complexes able to stabilize low oxidation state of Fe(I) and Co(I). The complexes were evaluated for antimicrobial and DPPH radical scavenging activities. They showed varying degree of activity with the values between 28.95-97.36 μm in DPPH radical scavenging activity. These synthesized metal complexes were found to be less active than the ligand and standard for DPPH radical scavenging activity. The antimicrobial activities of the metal complexes were found to more active than the ligand and the antifungal activity of the ligand, Fe(II) complex are insensitive.
Keywords
Itpy; DPPH; Antimicrobial; CHN elemental analysis; Cyclic voltammetry
Introduction
Imidazole and its derivatives form complexes with a number of transition metal ions. Imidazole is biologically the most important since the imidazole nitrogen of histidyl residues coordinate to metal ions in many metalloproteins. Azathioprine, dacarbazine and metronidazole are some examples of drugs possessing imidazole moiety. Imidazolyl terpyridine metal complexes have shown wide range of DNA binding behaviors and cytotoxic effects. Manikandamathavan and Balachandran Unni Nair have extensively investigated the DNA binding behaviours and cytotoxicity of copper (II) imidazolyl terpyridine complexes [1]. The same school of research has investigated the DNA/protein interaction and cytotoxic activity of imidazole terpyridine derived copper(II)/zinc(II) metal complexes [2]. Further Ramasamy Indumathy et al. [3] have synthesized and characterized imidazolyl terpyridine complexes with cobalt (II) and cobalt (III) ions. They also studied the CT DNA binding behavior and nuclease activity of these complexes. Iron is an essential element involved in an enormous range of functions. It is necessary for hemoglobin synthesis and oxidative processes of living tissues, as it exists at the active site of molecules responsible for oxygen transport and electron transport [4]. Since the first reported studies into the biological activity of Co complexes [5] in 1952, diverse structurally characterized cobalt complexes showing antitumor [6,7] antimicrobial [8,9] antifungal [10,11] antiviral [12,13] and antioxidant [14] activities have been reported. Hence in order to explore further the impact of imidazolyl substituent on the biological activities and coordination tendency of the terpyridine ligand system, we have synthesized 4'-(1H-imidazol-2-yl)-2,2’:6’,2’’-terpyridine and its metal (bivalent Fe, Co) complexes and studied their biological activities.
Experimental
Physical Parameters
C, H and N were estimated by using Elemental Vario EL III CHNS/O elemental analyser. The FT-IR and UV-vis spectra of itpy(L) and its metal complexes were recorded as KBR pellets in the range of 400-4000 cm-1 region using a Shimadzu FT-IR 8000 spectrophotometer and Perkin Elmer lambda 35 Uv-vis spectrophotometer in the region of 200-800 nm respectively. The ESI-MS spectra of the itpy and its metal complexes was recorded in Finnigan LCQ 6000 advantage max ion trap mass spectrometer equipped with an electron spray source. 1H NMR spectrum was recorded with a model Bruker Advance DPX-300 spectrometer operating at 300 MHz using CDCl3 as a solvent and TMS as an internal standard. The magnetic moments were carried out at room temperature by using a Gouy magnetic balance. Molar conductance was measured by DMF solutions at room temperature using a digital conductivity bridge, systronics direct reading conductivity meter 304 with a dip type conductivity cell. Cyclic voltammetric studies of the complexes were carried out by using three electrode systems in a single compartment comprising of glassy-carbon working electrode and potentials were referenced to standard calomel electrode.
Materials and Methods
2-acetyl pyridine, iron(II) perchlorate hexahydrate and cobalt(II) perchlorate hexahydrate were procured from Sigma Aldrich, USA and used as received. Other materials like sodium hydroxide, ammonium acetate and solvents like methanol, acetonitrile, ethonal, diethyl ether, and glacial acetic acid were of reagent grade.
Synthesis and Characterization of 4'-(1H-Imidazol-2-yl)-2,2’:6’,2’’-terpyridine
4'-(1H-imidazol-2-yl)-2, 2’:6’, 2’’-terpyridine (itpy) was synthesized using a slight modification of known procedure available in literature [15,16]. 2-Acetyl pyridine (1.12 ml, 10 mmol) and 1H-imidazol-2-carbaldehyde (0.73 g, 5 mmol) were intimately mixed by grinding the mixture in a mortar and pestle until the formation of an orange red powder. The powder was added to a suspension of ammonium acetate (2.5 g) in glacial acetic acid (10 ml) and heated to reflux for 3 hr. The crude product was precipitated by the addition of water (5 ml). The product was filtered, washed with water and then with cold ethanol. It was column chromatographed using silica gel and 1:1 methanoldichloromethane solvent system. (Yield: 1.43 g, 82%). Elemental analysis, found (calcd) for itpy: C, 71.93 (72.24); H, 4.22(4.35); N, 23.07(23.41); mass m/z=299.0.
Synthesis of Iron (II), Cobalt (II) Perchlorato Complexes with itpy
Complex 1
To the hot solution of hydrated Fe(ClO4)2 (50 mg, 0.196 mmol) in methanol, methanolic solution of itpy (117.49 mg, 0.39 mmol) was added slowly and the mixture was stirred for 1 h. The violet colored solid separated out upon slow evaporation of the solvent. It was filtered, washed with diethyl ether and then dried. Yield: 1.03 g, 81%. Elemental analysis, found (cal) for itpy: C, 49.95 (50.65); H, 2.94(3.05); N, 15.91(16.42); M, 6.40(6.55); mass, m/z=853.0.
Complex 2
To the hot solution of Co(ClO4)2.6H2O (100 mg, 0.273 mmol) in methanol, methanolic solution of itpy (163.6 mg, 0.55 mmol) was added slowly and the mixture was stirred for 1 h. A red brown solid crystallized on slow evaporation of the solvent. It was filtered, washed with diethyl ether and then dried. Yield: 1.0 g, 75%. Elemental analysis, found (cal) for itpy: C, 49.26 (50.47); H, 2.90(3.04); N, 17.02(16.36); M, 6.89(6.88); mass, m/z=855.9.
Antimicrobial Assay
The in vitro antimicrobial assay of the test drugs were tested for certain bacteria and fungi by using the Disc Diffusion Method and Mueller Hintonagar medium [17]. A loopful of strain in each was inoculated in 30 ml of the nutrient broth and incubated for 24 hours at 37°C to activate the strains. The dried surface of each agar-agar plate was swabbed with the respective suspension of the bacterial/fungal strain using a sterile cotton swab. The test drugs were dissolved in DMF to prepare stock solutions and concentrations of 100, 250 and 500 mg/ml were made by suitable dilutions. The sterilized filter paper discs were completely saturated with the test compounds and the impregnated dried discs were placed on the dried surface of inoculated agar plate. The agar plates inoculated with the bacterial organisms under test were incubated at 35°C to 37°C for 24 hours and the plates incubated with the fungi were incubated for 48 hours at the same temperature.
DPPH Radical Scavenging Assay
The free radical scavenging abilities of the terpyridyl ligand and its metal complexes have been determined by their interaction with the stable free radical 2, 2’-diphenyl-1-picryl hydrazyl [18-22]. The reduction capability of DPPH induced by antioxidants has been determined by the decrease in its absorbance measured at 517 nm.The sample solution in DMSO (5, 10, 15 or 20 μL) was mixed with 1 ml of 1.5 × 10-5 M DPPH solution in ethanol so as to make the desired concentrations of 5-20 μg/ml and the mixture have been kept in dark for 30 minutes. The quantity of DPPH remaining in each mixed solution has been determined by measuring the absorbance of mixed solution at 517 nm using a spectrophotometer. The decrease in the absorbance of DPPH in the mixed solution indicates the free radical scavenging activity of the test drug. L-ascorbic acid has been used as standard and ethanol has been employed as control.
Results and Discussion
Elemental Analyses
Elemental analyses give satisfactory results for ligand and its metal complexes. CHN values are in close agreement with expected molecular formulae assigned to the ligand and its metal complexes. The mass and elemental analysis data are presented in the experimental section. The IR and mass spectral data confirm that the structure of the ligand and complexes.
Mass Spectra and Molar Conductance
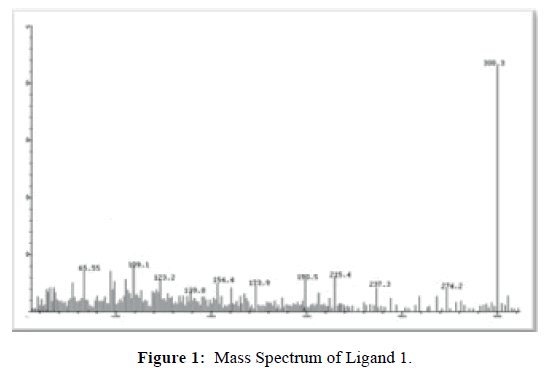
Ligand 1 shows its molecular ion peak at m/z=299.0 (Figure 1) which confirms it molecular mass and molecular formula. The mass spectrum of Complex 1 (Figure 2) shows its molecular ion peak at m/z=853. The complex measure molar conductance at 32.40 S cm2mol-1 in DMF solution indicating the non-electrolytic nature [23] of the complex due to the coordination of perchlorate anions to the metal centre. Complex 2, exhibits its molecular ion peak at m/ z=855.9 and measure a molar conductance in DMF solution at 34.36 S cm2mol-1 predicting that the perchlorate ions have coordinated to the cobalt (II) ion and that the complex is non-ionic in nature [23].
1HNMR Spectral Study on Imidazolylterpyridine (itpy)
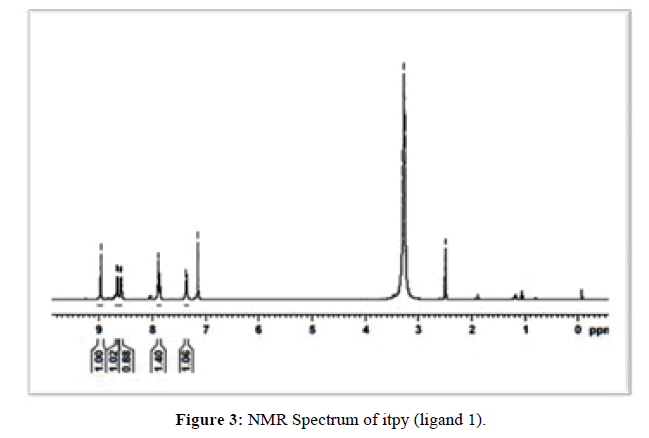
The proton NMR spectrum of itpy (L1) recorded in CDCl3 is reproduced in (Figure 3) and the chemical shifts and their assignments are furnished in (Table 1).
| Sl.No | Chemical shift | Assignment |
|---|---|---|
| 1 | 7.151 | H5/H5’’ of tpy moiety |
| 2 | 7.352 - 7.382 | H3’/H5’ of tpy moiety |
| 3 | 7.863 | H4’/H4’’ of tpy moiety |
| 4 | 7.882 - 7.901 | CH protons of imidazole |
| 5 | 8.582 - 8.602 | H3/H3’’ of tpy moiety |
| 6 | 8.660 - 8.670 | H6/H6’’ of tpy moiety |
| 7 | 8.975 | NH proton of imidazole |
Table 1: 1H NMR Chemical Shifts (δ ppm) for L1.
The 1HNMR chemical shifts of itpy have been assigned on the basis of data provided by Elsbernd and Beattie [24]. Protons at 3, 4, 5 and 6 positions and the symmetrically related proteins at 3’’, 4’’, 5’’ and 6’’ protons form a 4-spin system and central ring protons at 3’, 4’, and 5’ form a 3-spin system in the NMR terpyridine. It is to be noted that in the case of itpy also the chemical shifts of terminal ring protons are independent of the nature of 4’-substituent [25-26] (i.e. imidazole ring). The aromatic CH protons of the imidazole ring absorb at 7.882-7.901 ppm and NH proton of the imidazole ring resonates at 8.975 ppm. The absorption at 2.495-2.503 ppm and 3.278 ppm may be considered to be due to impurities in the solvent. Hence based on elemental analysis mass, IR, UV and 1HNMR spectral data the structure of 4'-(1H-imidazol-2-yl)-2,2':6',2''-terpyridine (itpy) has been confirmed.
Electronic Spectral and Magnetic Studies on itpy (L1) and its Metal Complexes
The L1 consist of two bands at 217 nm and 315 nm. These bands are assigned to π - π* and n - π* transition. The electronic spectra of the complex 1 showed two bands at 12850 cm-1(single d-d transition) and 17376 cm-1. These bands are assigned to 5T2g → 5Eg [27-30] and charge transfer band [27]. The magnetic moment of complex 1 was found to be 5.3 BM [30]. These electronic spectra and magnetic value proposed octahedral environment around the metal ion. On the other hand, the electronic spectra of the complex 2 showed three bands at 17331 cm-1 (ν 3), 20150 cm-1 (ν 2) and 30490 cm-1. The first two bands are assigned to 5T1g (F) → 4T1g (P), 4T1g (F) → 4A2g (F). The third band is assigned to M-L charge transfer. The magnetic moment of the Complex 2 was found to be 4.6 BM [28-31]. These results are typically characteristic for an octahedral configuration for the cobalt complex.
IR spectroscopy
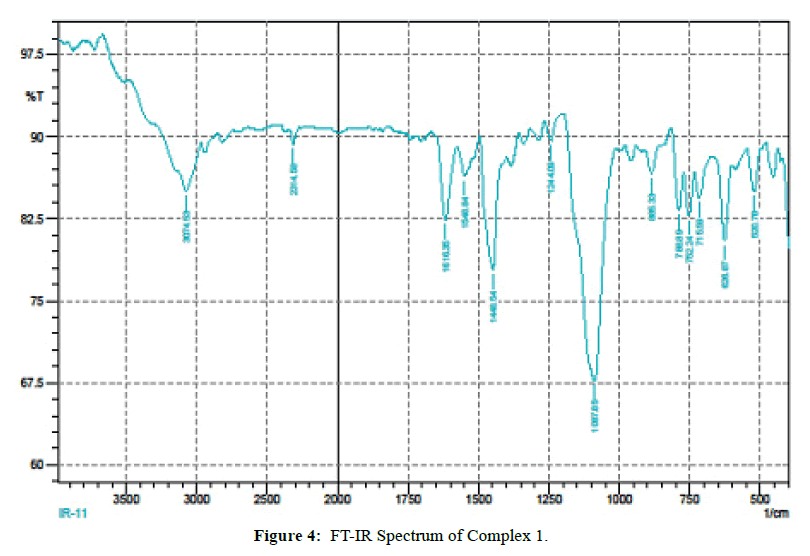
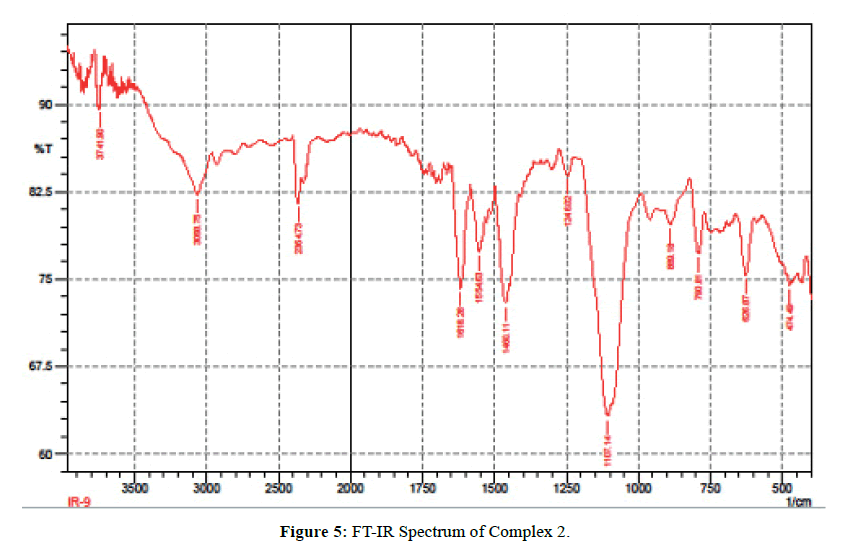
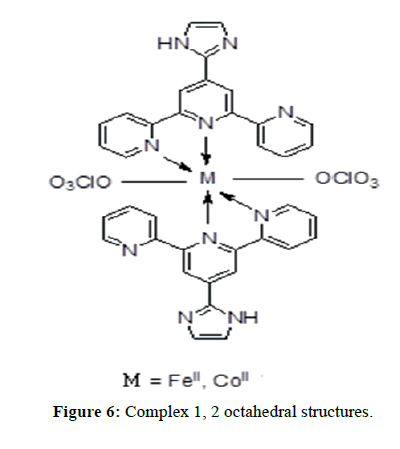
The IR spectra of itpy(L) and and its metal complexes(Figures 4 and 5) exhibit several diagnostic vibrational frequencies of respective functional groups. The characteristic stretching details are summarized in (Table 2). IR spectrum of L1 displays bands at 3300, 3013-2772, 1555-1437, 621 and 430 cm-1 assigned to ν(NH), ν(CH), pyridine ring skeletal vibrations (νC=C and νC=N), in-plane and out-of- plane deformations respectively. The Complex 1, 2 νC=C and νC=N bands have shifted to higher position (1616-1449 cm-1) and (1618-1460 cm-1) due to the coordination of the pyridyl nitrogen atoms to the metal ion [31]. The pyridine ring in-plane deformation and out-of-plane deformation confirming the coordination of two nitrogen atoms at 1’ and 1’ positions [32-34]. The two new bands at 550, 474 and 521, 450 cm-1 were also being observed in the complexes and were not found in the free ligand. These bands attributed to M-N and M-O bonds in complexes 1-2 [35] (Table 2) respectively. Based on these data, Complex 1, 2 proposed to have octahedral structure (Figure 6).
| Sl. No. | Compound | νNH | νCH | pyridine ring skeletal vibration | pyridine ring In plane Deformation |
pyridine ring out-of-plane deformation |
νClO | νMO and νMN |
|---|---|---|---|---|---|---|---|---|
| 1 | itpy (L) | 3300 | 3013-2772 | 1555-1437 | 621 | 430 | - | - |
| 2 | [Fe(itpy)2(ClO4)2] Complex 1 |
3400 | 3074 | 1616-1449 | 627 | 460 | 1244 1088 |
550 521 |
| 3 | [Co(itpy)2(ClO4)2] Complex 2 |
- | 3069 | 1618- 1460 |
627 | 450 | 1246 1107 |
474 450 |
Table 2: Selected IR Absorptions for itpy (L1) and its Metal Complexes.
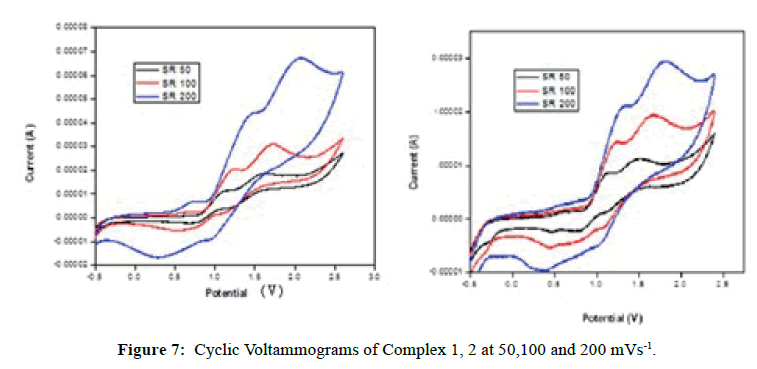
Cyclic Voltammetry
To investigate the redox properties of the complexes, the cyclic voltammograms were recorded for the complexes in DMSO using the same cell set up. The cyclic voltammograms of the complexes have been measured at 50, 100 and 200 mVs-1 scan rates (Figure 7). Complex 1, the Epa represents the energy required for oxidation of the central metal ion and the Epc represents energy needed for reduction of the metal ion at a particular scan rate. The Epc values of 1.015, 0.992 and 1.584 V feature the reduction of FeI to FeII species. Reoxidation of FeI to FeII species has happened at Epa values of 1.607, 1.709 and 2.062 V [36,37]. The ΔEp between peak potentials are higher than 200 mV indicating an irreversible one electron redox couple of FeII/FeI. The E1/2 indicate that the FeII species cannot be reduced easily due to stronger σ-donating ability of ligand. The peak current ratios are less than unity suggesting that electron transfer is followed by a chemical reaction.
Complex 2 undergoes reduction to CoI species at cathodic peak potentials of 1.033, 1.487 and 1.441 V. But the reduced form of CoI species reoxidizes to CoII species at the respective anodic peak potentials of 1.501, 1.688 and 1.809 V. The ΔEp values higher than 200 mV which reveal that an irreversible one electron redox couple of CoII/CoI exits [36,37]. The peak current ratios less than unity suggesting that the electron transfer is followed by a chemical reaction. The Ipa values have increased with increase of ν1/2 to suggest that the redox process is diffusion control. The E1/2 values are all positive to show that the complex 2 may not undergo easy reduction as it is stabilized by the higher σ- donating ability of the ligand.
Biological Screening
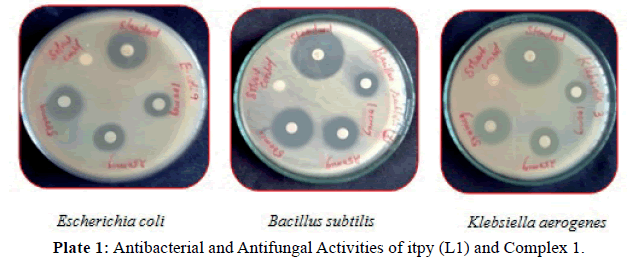
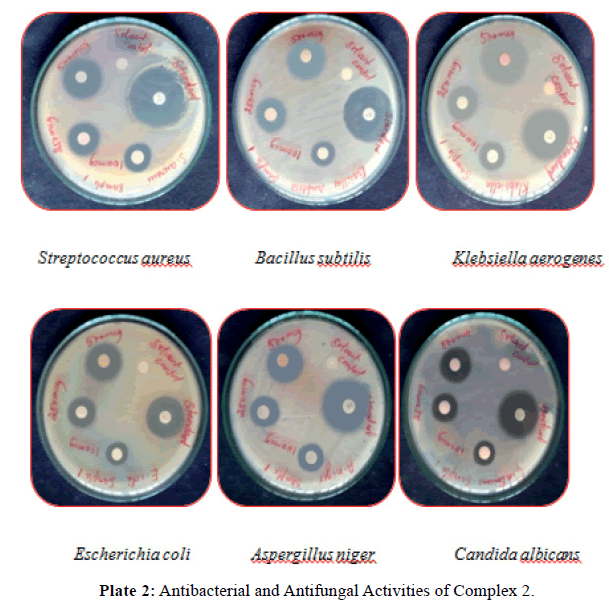
Antimicrobial activity: The results of antibacterial, antifungal activities of itpy (L1) and its metal complexes in comparison with that of the standard drug (ciprofloxacin, nystatin) are furnished in (Tables 3 and 4) and (Plates 1, 2). From the result we can see that itpy showed very little antimicrobial activity against E.coli, and insensitive against S.aureus, B.subtilis, K.aerogenes, A.niger and C.albicans. Complex 1 was insensitive against S.aureus, E.coli, A.niger and C.albicans and little active against B.subtilis, K.aerogenes. The cobalt complex 2 exhibits a significant increase activity against S.aureus, B.subtilis, K.aerogenes and moderate activity against E.coli, A.niger and C.albicans.
| Sl. No |
Test Drug | Zone of Inhibition (mm) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus | B. subtilis | K. aerogens | E. coli | ||||||||||
| 100 mg/L |
250 mg/L |
500 mg/L |
100 mg/L |
250 mg/L |
500 mg/L |
100 mg/L |
250 mg/L |
500 mg/L |
100 mg/L |
250 mg/L |
500 mg/L |
||
| 1 | itpy(L1) | - | - | - | - | - | - | - | - | - | 10 | 10 | 16 |
| 2 | [Fe(itpy)2(ClO4)2] Complex 1 |
- | - | - | 8 | 10 | 15 | 12 | 15 | 18 | - | - | - |
| 3 | [Co(itpy)2(ClO4)2] Complex 2 |
15 | 16 | 20 | 16 | 18 | 22 | 15 | 19 | 22 | 12 | 15 | 16 |
| 4 | Ciprofloxacin Standard |
- | - | 35 | - | - | 40 | - | - | 30 | - | - | 38 |
Table 3: Antibacterial Activities of itpy (L1) and its Metal Complexes.
| Sl. No | Test Drug | Zone of Inhibition (mm) | |||||
|---|---|---|---|---|---|---|---|
| Aspergillus niger | Candida allbicans | ||||||
| 100 mg/L |
250 mg/L |
500 mg/L |
100 mg/L |
250 mg/L |
500 mg/L |
||
| 1 | itpy(L1) | - | - | - | - | - | - |
| 2 | [Fe(itpy)2(ClO4)2] Complex 1 |
- | - | - | - | - | - |
| 3 | [Co(itpy)2(ClO4)2] Complex 2 |
15 | 16 | 18 | 14 | 14 | 18 |
| 4 | Nystatin (Standard) | - | - | 30 | - | - | 25 |
Table 4: Antifungal Activities of itpy and its Metal Complexes.
Antioxidant activity screening
The itpy and its metal complexes were evaluated for DPPH radical scavenging activity [38,39]. The antioxidant activity of each test drug increases with increase in its concentration. The determined results show that the itpy (L1) exhibits the highest activity at 97.36% while the FeII complex displays the lowest activity at 73.68% at the concentration of 20 μg/ml.The decreasing order of antioxidant activities is itpy > Complex 2 > Complex 1.
Conclusion
In this study, we have reported the synthesis of itpy ligand and its Fe(II), Co(II) complexes. The structural characterizations of the synthesized compounds were made by spectroscopic methods (FT-IR, Electronic, 1H NMR, ESI-MS), elemental analysis, electrical conductance, magnetic studies and cyclic voltammetry. From the spectroscopic characterization, it is concluded that itpy act as bidentate ligands, coordinating through its two 1, 1’ positions to the metal. The redox properties of these complexes are able to stabilize the +1 oxidation state of iron and Cobalt. The biological activity screening showed that the complexes selectively inhibited the bacterial (S.aureus, B.subtilis, K.aerogenes and E.coli) and fungal (A.niger, C.albicans), the cobalt complex has more activity than iron complex and the ligand. The antioxidant properties of ligand and complexes have been tested using DPPH radical scavenging method in which cobalt complex exhibited potential antioxidant capacity than that of ligand and iron complex. However, further studies on the mechanisms of antioxidant are required.
Acknowledgement
The authors are thankful to SAIF-IIT Chennai, SMS-EUMIC lab, Periyar Maniyammai Pharmaceutical College – Tiruchirappalli, India.
References
- Manikandamathavan VM, Nair BU (2013) DNA Bind and Cytotoxicity of Cu(II) Imidazole terpy Comp; Role of Oxyanion, Hydrogen bonding and π-π interaction. Eur J Med Chem 68: 244-252.
- Manikandamathavan VM, Weyhermuller T, Parameswari PR, Sathishkumar M, Subramanian V, et al. (2014) DNA Protein Interaction and Cytotoxic Activity of Imidazole Terpy. Deri.Cu, Zn metal comp. Dalton Trans, 43:13018-13031.
- Indhumathy R, Kanthimathi M, Weyhermuller T, Nair BU(2008) Cobalt Comp. of Terpy Ligand Crystal Structure and Nuclease Activity, Polyhedron 27: 3443-3450.
- Cotton FA, Wilkinson G (1998) The Biochemistry of Iron in Advanced InorganicChemistry, 5th ed., Wiley Interscience, USA, p.1336
- Hall MD, Failes TW, Yamamoto N, Hambley TW (2007) Bioreductive Activation and Drug Chaperoning in Co Pharmaceuticals. Dalton Trans 36: 3983-3990.
- Sandoval HL, L-Lemos ME, Velasco RG, Melendez IP, Macias PG et al. (2008) Synthesis, structure and Biological Activities of Co and Zn Coor. Compounds with Benzimidazole Derivatives. J Inorg Biochem 102: 1267-1276.
- Ott I, Abraham A, Schumacher P, Shorafa H, Gasti G, et al. (2006) Synergistic and Additive Antiproliferative Eff. on Human Leukemia Cell lines induced by combining Acetylienehexa carbonylidicobalt Complexes with the tyrosinekinase Inhib. Imatinib. J Inorg Biochem 100: 1903-1906.
- Miodragovic DU, Bogdanovic GA, Miodragovic ZM, Radulovic MD, Novakovic SB, et al. (2006) Interesting Coordination Abilities of Antiulcer Drug Famotidine and Anti. Microbiol Activity of drug and its Co Complexes. J Inorg Biochem 100: 1568-1574.
- Nomiya K, Yoshizawa A, Tsukagoshi K, Kasuga NC, Hirakawa S, et al. (2004) Synthesis and Structural Characteristics of Ag, Al, Co complexes with 4- isopropyltropolone showing noteworthy Biological activities. J Inorg Biochem 98: 46-60.
- Lu J, Liu T, Cai S, Wang X, Liu L, et al. (2006) Synthesis, structure and Biological Activity of Co, Cu Complexes of Valine derived Schiff bases. J Inorg Biochem 100: 1888-1896.
- Weiqun Z, Wen Y, Liqun X, Xianchen C (2005) N-benzoyl-N-Dialkylthiourea derivatives and their Co complexes; Str. and Antifungal. J Inorg Biochem 99: 1314-1319.
- Bottcher A, Takeuchi T, Hardcastle KI, Meade TJ, Gray HB (1997) Spectroscopy and Electrochemistry of Co Schiff base Complexes. Inorg Chem 36(12): 2498-2504.
- Takeuchi T, Bottcher A, Quezada CM, Meade TJ, Gray HB (1999) Inhibition of Thermolysin and Human α-thrombin by Co Schiff base Complexes. Bioorg Med Chem 7: 815-819.
- Dimiza F, Papadopoulos AN, Tangoulis V, Psycharis V, Raptopoulou CP, et al. (2010) Biological evaluation of non-steroidal anti-inflammatory drugs- Co Complexes. Dalton Trans 39: 4517-4528.
- Cargill Thompson AMW (1997) The Synthesis of 2,2’;6’2’’-tepy ligands-Versatile building blocks for Sup Mole Chem Coord Chem Rev 160: 1-52.
- Cave GWV, Raston CL (2001) Effi Synthesis of Pyridine via a Sequential solvent less aldol Condensation and Michael Addition. J Chem Soc Perkin Trans 1: 3258-3264.
- Verma RS, Nobles WL (1975) Antiviral, antibacterial and antifungal activities of isatin-N- Mannich bases. J Pharm Sci 64: 881-882.
- Ancerewicz J, Migliavacca E, Carrupt PA, Testa B, Dree FJ, et al. (1998) Structure Prop relationships of trimetazidine derivatives and model compounds as potential antioxidants. Free Radical Biol Med 25: 113-120.
- Karki SB, Treemaneekam V, Kaufman MJ (2000) Oxidation of HMG-CoA reductase Inhib. By Tert-butoxyl and DPPH radicals; model reactions for predicting oxidatively sensitive compound during preformulation. J Pharm Sci 89: 1518-1524.
- Moridani MY, Pourahmad J, Bui H, Siraki A, O’Brien PJ (2003) Dietary flavonoid Fe complexes as cytoprofective superoxide radical scavengers. Free Radical Biol Med 34: 243-253.
- Oktay M, Gulcin I, Kufrevioglu OI (2003) Determination of invitro antioxidant activity of Fennel seed extracts. Liebensm Wiss U Technol 36: 263-271.
- Saito S, Kawabata J (2005) Effect of electron-withdrawing substance on DPPH Radi scavenging rea of protocatechuic acid its analogues in alcoholic solvents. Tetrahedron 61: 8101-8108.
- Geary WJ (1971) The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord Chem Rev 7: 81-122.
- Elsbernd H, Beattie JK (1972) The NMR Spectra of terpy and the bis terp and bis terpy. Complexes of Co and Fe. 34: 771-774.
- Constable EC, Cargill Thompson AMW, Tocher DA (1992) Supramolecular Chemistry, Eds. V Balzani and L DeCola, Kluwer, Academic Publishers, Dordrecht.
- Constable EC (1994) Transition Metals in Supramolecular Chemistry, Eds. L Fabbrizzi, A Poggi, Kluver, Dordrecht.
- Nakamato K (1978)Infrared and Raman Spectra of Inorganic and Coordination Compounds. Wiley Interscience, New York.
- Lever ABP (2000) Inorganic Electronic Spectroscopy. Elsevier, Amsterdam.
- Drago RS (1978) Physical Methods in Inorganic Chemistry. East West Press, New Delhi.
- Figgis BN (1976) Introduction to Ligand Fields. Wiley Eastern Ltd, New Delhi.
- Cotton FA, Wilkinson G (1972)Advanced Inorganic Chemistry. Wiley Interscience, New York.
- Mallet C, Thummel RP, Hery C (1993) The conformational Proproperty of Eu complexes of polymethylene bridged derivatives of tepy. Inorg Chim Acta 210: 223-231.
- Constable EC, Cargill Thompson AMW (1994) Novel didentate tepy Complexes of Ru. Inorg Chim Acta 223: 177-179.
- Aimeloglou N, Michael PKB, Drew GB, Glaeser B, Holland F, et al. (2000) 2,2’;6’,2’’-Tepy. Complexes of Mo and W. J Organomet Chem 604: 191-196.
- Ferraro JR (1971) Low Freguency Vibration of Inorganic and Coordination Compounds. Plenum Press, New York.
- Bard AJ, Isata LR (2001) Electrochemical Methods, Fundamentals and Applications. Wiley, New York.
- Bard AJ, Faulkner LR (1980) Electrochemical Methods, Fundamentals and Applications. John Wiley and Sons, USA. pp:193-197.
- Wu HC, Chen HM, Shiau CY (2003) Free Amino acids and peptides as related to antioxidant prop in protein hydrolysates of mackerel. Food Res Int 36: 949-957.
- Ancerewicz J, Migliavacca E, Carrupt PA, Testa B, Dree F, et al. (1998) Structure Prop Relation of trimetazidine derivatives and model compound as potential antioxidants. Free Radical Biol Med 25: 113-120.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences