ISSN : 0976-8505
Der Chemica Sinica
Synthesis, Spectral Characterization, DFT Calculations and In Silico ADMET Study of E-(Naphthalen-6-yl) Methylene) Semicarbazide (NMS)
Rajalakshmi R*, Theresa AA and Ramkumar S
Department of chemistry, Annamalai University, Tamil Nadu, India
- *Corresponding Author:
- Rajalakshmi R
Department of chemistry,
Annamalai University, Tamil Nadu,
India,
Tel: +91 8838866540;
E-mail: chemrajalaksmi@gmail.com
Received date: August 12, 2022, Manuscript No. IPDCS-22-14329; Editor assigned date: August 15, 2022, PreQC No. IPDCS-22-14329 (PQ); Reviewed date: August 31, 2022, QC No. IPDCS-22-14329; Revised date: September 07, 2022, Manuscript No. IPDCS-22-14329 (R); Published date: September 15, 2022, DOI: 10.36648/0976-8505.13.8.56
Citation: Rajalakshmi R, Alphonsa AT, Ramkumar S (2022) Synthesis, Spectral Characterization, DFT Calculations and In Silico ADMET Study of E- (Naphthalen-6-yl) Methylene) Semicarbazide (NMS). Der Chem Sin Vol.13 No.8: 056.
Abstract
The Schiff base (Naphthalen-6-yl) Methylene) Semi carbazide (NMS) is synthesized and characterised using FTIR, 1H NMR and 13C NMR spectral methods. A molecular docking experiment was utilised to determine the ADME properties of NMS and forecast their interactions with the oestrogen receptor in order to identify the lead chemical (2IOK).In the ultra-violet absorption spectrum, the substance exhibits absorption at 396 nm. Density functional theory was used to compute the electronic states and molecular characteristics of the molecule.
Keywords
Schiff base; Auto dock; DFT.
Introduction
Schiff base-based metal complexes have received a lot of attention due to their biological activities. Numerous derivatives of the Schiff bases have been created and used to create protein and enzyme imitators [1].
Investigations into the structure can help us to understand the co-ordination characteristics of Schiff bases acting as ligands. The use of Schiff base ligands in inorganic chemistry has been the subject of substantial study throughout the past thirty years. This has led to reports that several of these species make for excellent reagents in biological, pharmacological, therapeutic and analytical applications. We describe here the synthesis and molecular structure of the above mentioned chemical as part of an inquiry into their crystal structures that will help shed light on the coordination characteristics of Schiff bases acting as ligands [2,3].
Nuclear hormone receptors such as the Oestrogen Receptor (ER) bind to DNA and regulate a number of gene-related processes. Antiestrogens, or ER (Estrogen Receptor) blockers, stop the growth of tumours. Numerous antiestrogens, such as bazedoxifene, clomifene, cyclofenil, epimestrol, lasofoxifene, ormeloxifene, raloxifene, tamoxifen and toremifene, have been developed as medications to block the oestrogen signal [3-6]. A potential first step in the creation of cutting-edge and strong anticancer drugs with significant cytotoxic activity against breast and ovarian cancer is represented by symmetrical azine derivatives [6-9]. Density Functional Theory (DFT) computations and spectroscopic investigations are essential to determine the structural link between the groups that affect biological features [9-15].
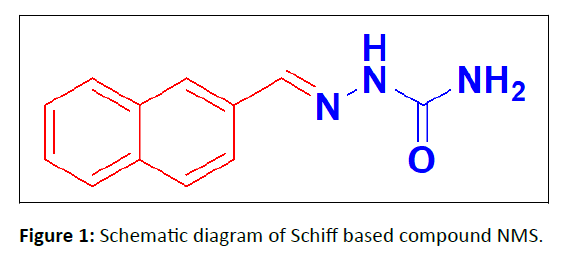
In the present study, we report the synthesis and molecular structure of a (Naphthalen-6-yl) Methylene) Semi carbazide (NMS). It is a Schiff base derivative and its schematic diagram is shown in Figure 1.
Experimental
Synthesis
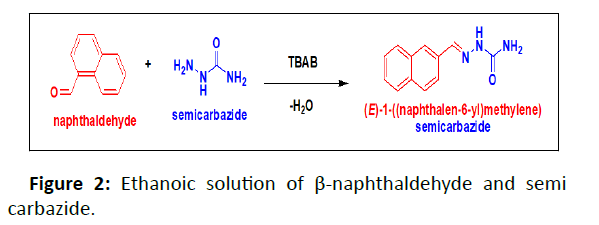
The ethanoic solution of β-naphthaldehyde (0.4074 g) and semi carbazide (0.4985 g) were stirred with small quantity of Tetra Butyl Ammonium Bromide (TBAB) solution for 4hrs. The resulting solution is poured into ice-water and the precipitate formed was filtered and recrystallized from ethanol (Figure 2).
Results and Discussion
FT-IR
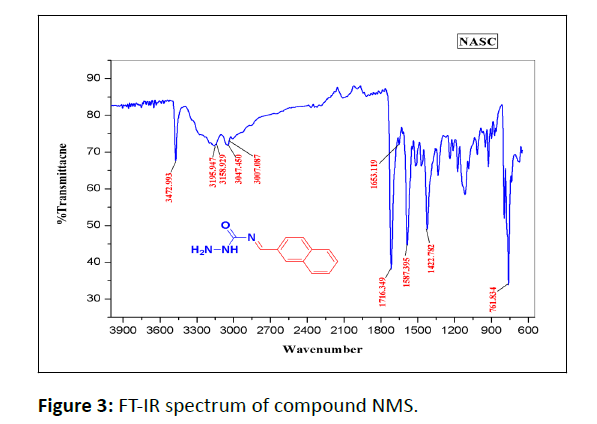
In the IR Spectrum of compound NMS shown in (Figure 3).
The ˃C=O˂ stretching vibration frequency is observed at 1716 cm-1. The peaks at around 1587 cm-1 is due to the ˃C=C˂ stretching vibration. >C=N stretching vibration appear at 1653 cm-1.
Ultraviolet absorption spectrum
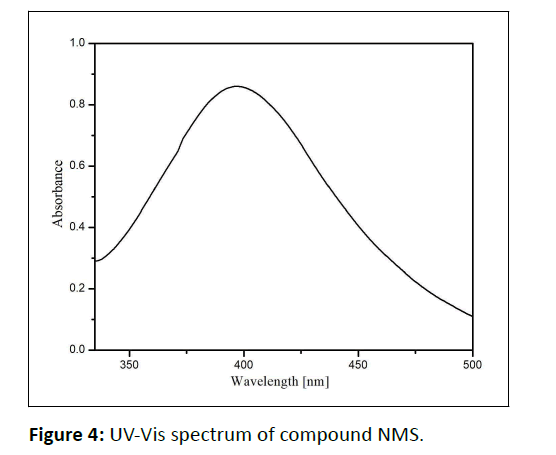
The ultraviolet absorption spectra are recorded for the newly synthesized symmetrical azine in ethanol the absorption in 395.5 nm might due to the n- π * transition shown in Figure 4.
NMR analysis
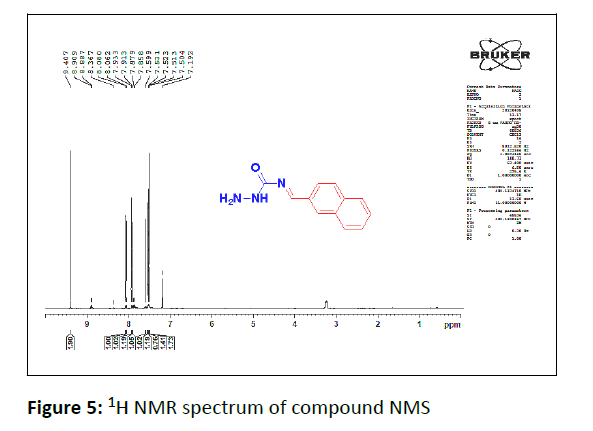
1H NMR
In the 1H NMR spectrum of compound NMS. The two protons singlet appears at 9.40 ppm are due to the NH2 protons. The benzylidine proton appears as singlet at 8.36 ppm. The NH proton resonates as a singlet at 7.19 ppm. The protons signal ranging from 7.50 ppm-8.0 ppm is obviously due to the aromatic protons (Figure 5).
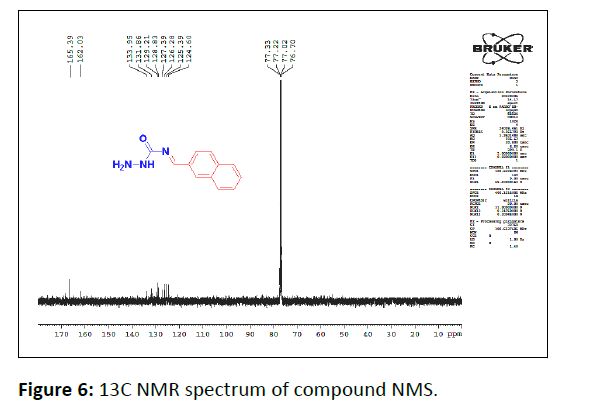
13C NMR
In the 13C NMR spectrum of compound the carbons signals at 165.39 ppm and 162.03 ppm is due to the carbonyl carbon and benzylidine carbon respectively. The carbon signal ranging from 124 ppm-133 ppm is due to the aromatic carbons Figure 6.
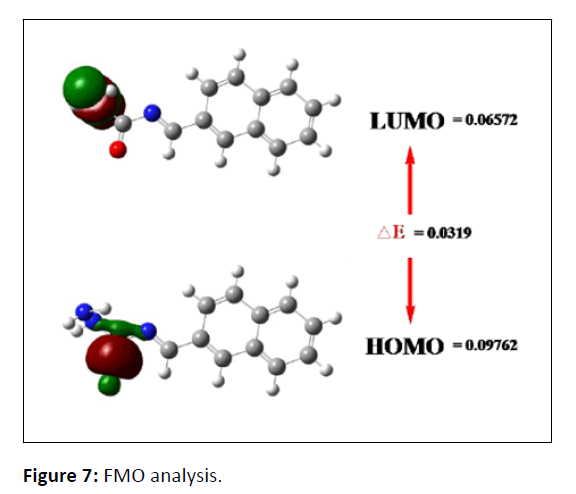
Frontier Molecular Orbital analysis (FMO)
The border orbital gap can be used to explain more clearly the chemical reactivity and kinetic stability of a molecule. An electron donor is present in the HOMO molecular orbital, which has the highest density of occupants, while an electron acceptor is present in the LUMO, which has the lowest density of occupants. The base set B3LYP was used in the calculations. The HOMO-LUMO gap of 0.0319 eV and the characteristics of the molecule indicate that it is soft, reactive and polarizable (Figure 7). Table 1 displays the findings of the compound NMS FMO Analysis.
| Properties | NMS |
|---|---|
| EHOMO (eV) | -0.09762 |
| ELUMO(eV) | -0.06572 |
| ∆ E (EHOMO-ELUMO) eV | 0.319 |
| Global hardness (ɳ) (∆ E/2) | 0.01595 |
| Softness (S) (1/ ∆ E = 1/2 ɳ) | 31.3479 |
| Chemical Potential (µ) (–[1/2 EHOMO + ELUMO]) | 0.08167 |
| Electrophilicity (Ͳ) µ 2/2 ɳ | 0.41818 |
| Electronegativity (χ) (- µ) | -0.08167 |
| Dipolemoment (Debye) | 1.742 |
Table 1: FMO Analysis of compound NMS.
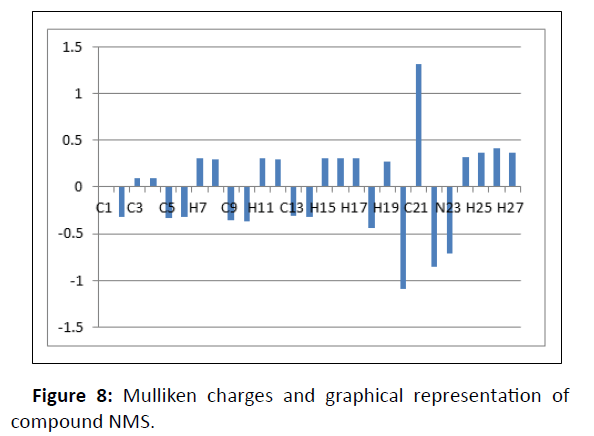
Mulliken charge distribution
It explains how charges are distributed throughout the different molecular orbital subshells (core, valance and Rydberg). Table 2 displays the buildup of natural charges on particular title-molecule atoms. Quantum chemistry computations of molecular systems depend on the Mulliken atomic charge calculation. Atomic charge has an impact on the system's dipole moment, polariz ability, electronic structure, as well as other molecular characteristics. In Figure 8, the Mulliken charges of atoms were graphically represented.
| C1 | 0.014 |
| C2 | -0.323 |
| C3 | 0.093 |
| C4 | 0.092 |
| C5 | -0.328 |
| C6 | -0.319 |
| H7 | 0.304 |
| H8 | 0.301 |
| C9 | -0.353 |
| C10 | -0.364 |
| H11 | 0.306 |
| H12 | 0.296 |
| C13 | -0.313 |
| C14 | -0.319 |
| H15 | 0.304 |
| H16 | 0.309 |
| H17 | 0.309 |
| C18 | -0.434 |
| H19 | 0.275 |
| N20 | -1.09 |
| C21 | 1.319 |
| O22 | -0.848 |
| N23 | -0.707 |
| N24 | 0.318 |
| H25 | 0.367 |
| H26 | 0.409 |
| H27 | 0.368 |
Table 2: Mulliken charges of compound NMS.
Molecular docking
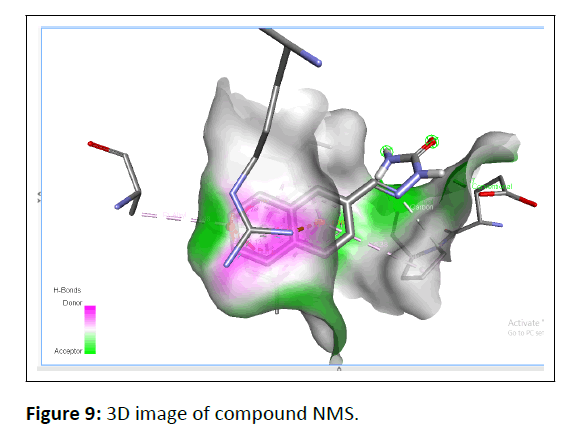
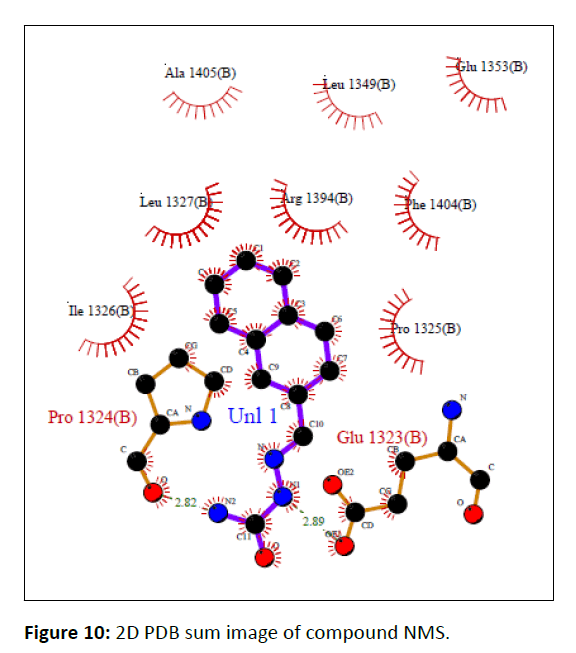
In silico molecular docking was used to identify the optimum molecules and molecular interactions for symmetrical azines. Before being used in molecular docking study to ascertain the compounds' binding interactions, the Human Estrogen Receptor Alpha Ligand-Binding Domain in Association with Compound 1D (PDB: 2IOK) X-ray crystal structures were retrieved from Protein Data Bank and modified. Figures 9 and 10 display the crucial residues in the binding region together with the best-scoring docking study pose for the compound NMS.
The docking investigations revealed that the active chemical NMS had the lowest binding energy, with a value of -6.88. According to glide molecular docking, Table 3 depicts the contact between the protein ligand and the ER as having the least amount of binding energy (PDB: 2IOK).
| Ligand | CAHD |
|---|---|
| Binding energy | -6.88 |
| Ligand efficiency | -0.43 |
| Inhib_Constant | 9.13µM |
| Intermol_energy | -7.47 |
| Vdw_hb_disolve_energy | -7.34 |
| Electrostatic energy | -0.13 |
| Total_internal | -0.21 |
| Torsional energy | 0.6 |
| Unbound energy | 0.21 |
| refRMS | 61.28 |
Table 3: Lowest binding energy for the ligand NMS and ER protein (PDB: 2IOK).
ADME
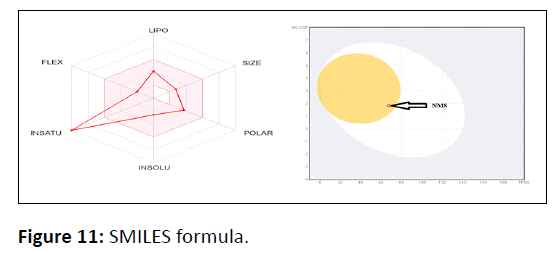
Swiss ADME software (www.swissadme.ch) from the Swiss Institute of Bioinformatics (http://www.sib.swiss) was used to estimate individual ADME behaviours of symmetrical azine compounds in a web server that displays the Swiss ADME Submission page in Google. Simplified Molecular Input Line Entry System (SMILES) defines the list which has one input molecule per line with multiple inputs and the results are provided in Tables 4,5,6 for each molecule Figure 11.
| Molecule | Molecular formula | Molecular weight g/mol | No.of hydrogen bond acceptor | No.Of hydrogen bond donor | Lipophilicity Log Po/w |
|---|---|---|---|---|---|
| NMS | C12H11N3O | 213.24 | 2 | 2 | 1.68 |
Table 4: Lipophilicity of the compound NMS.
| Water solubility | ||||||
|---|---|---|---|---|---|---|
| Compound | Log S (ESOL) | Class | Log S (Ali) | Class | Log S (SILICO-IT) | Class |
| NMS | -2.59 | Soluble | -2.89 | Soluble | -3.81 | Soluble |
Table 5: Water solubility of the compound NMS.
| Compound | Lipinski | Bio avalailability | Pharmacokinetics | Synthetic accessibility | |
|---|---|---|---|---|---|
| GI absorption | Log KP (Skin permeation) | ||||
| NMS | Yes (Zero violation) | 0.55 | High | -6.29 cm/s | 2.18 |
Table 6: ADME properties of the compound NMS.
SMILES formula: NC(=O)N\N=C\C1=CC2=C(C=CC=C2)C=C1
Conclusion
The Schiff’s base NMS was synthesized and thoroughly characterized using FT-IR, UV and NMR with all of the results supporting the predicted structures. According to the docking tests the active compound has the lowest binding energy with -6.88. Furthermore the compounds had favorable pharmacokinetic qualities and adhered to Lipinski's rule of five. 6-311(d,p) was also used to optimize the geometrical properties of the compounds. The stability, intermolecular charge transfers and donor-acceptor interactions in the synthesized molecule are clearly supported by DFT. The nucleophilic and electrophilic areas of the molecular surface were investigated using Mulliken atomic charges.
References
- Kahwa IA, Selbin J, Hsieh TCY, Laine RA (1986) Synthesis of homodinuclear macrocyclic complexes oflanthanides and phenolic schiff bases. Inorganica Chimica Acta 118: 179-185.
[Crossref], [Google Scholar]
- Santos MLP, Bagatin IA, Pereira EM, Ferreira AMDC (2001) Redox behaviour and reactivity of some di-Schiff base copper (II) complexes towards reduced oxygen species. Journal of the Chemical Society, Dalton Transactions 838-844.
[Crossref], [Google Scholar]
- Wang Y, Yang ZY, Wang BD (2005) Synthesis, characterization and anti-oxidative activity of Cobalt(II), Nickel(II) and Iron(II) Schiff base complexes. Transition Met Chem 7: 879-883.
[Crossref], [Google Scholar]
- Arruebo M, Vilaboa N, Sáez-Gutierrez B, Lambea J, Tres A, et al. (2011) Assessment of the evolution of cancer treatment therapies. Cancers 3(3): 3279-3330.
[Crossref], [Google Scholar], [Indexed]
- Maruthanila VL, Elancheran R, Kunnumakkara AB, Kabilan S, Kotoky J (2017) Recent development of targeted approaches for the treatment of breast cancer. Breast Cancer 24(2): 191-219.
[Crossref], [Google Scholar], [Indexed]
- Maximov PY, Lee TM, Jordan VC (2013) The discovery and development of selective estrogen receptor modulators (SERMs) for clinical practice. Curr Clin Pharmacol 8(2): 135-155.
[Crossref], [Google Scholar], [Indexed]
- Kurteva VB, Svilen PS, Margarita SD (2011) Symmetrical acyclic aryl aldazines with antibacterial and antifungal activity. Pharmacology & Pharmacy 2: 1.
[Crossref], [Google Scholar]
- Sundar S, Ramesh R, David S (2020) Non pincer type Arene Ru (II) catalysts for the direct synthesis of azines from alcohols and hydrazine under Aerobic conditions. Organometallics 39(17): 3194-3201.
[Crossref], [Google Scholar]
- Arjun HA, Elancheran R, Manikandan N, Lakshmithendral K, Manathan MR, et al. (2019) Design, synthesis and biological evaluation of (E)-N’-((1-chloro-3,4-dihydronaphthalen-2- yl)methylene) benzohydrazide derivatives as anti-prostate cancer agents. Front Chem 7: 474.
[Crossref], [Google Scholar], [Indexed]
- Devi KS, Subramani P, Sundaraganesan N, Jeeva M, Pradeepa SJ, et al. (2021) Synthesis, spectra, electronic structure, molecular docking and cytotoxicity investigation on 2-(piperidin-1-ylmethyl)-isoindoline-1, 3-dione- A Mannich base system. J Mol Struct 1224: 129151.
[Crossref], [Google Scholar]
- Elancheran R, Saravanan K, Divakar S, Kumari S, Maruthanila VL, et al. (2017) Design, synthesis and biological evaluation of novel 1, 3-thiazolidine-2, 4-diones as anti-prostate cancer agents anticancer agents. Med Chem 17: 1756-1768.
[Crossref], [Google Scholar], [Indexed]
- Douche D, Sert Y, Brandán SA, Kawther AA, Bilmez B, et al. (2021) 5-((1H-imidazol-1-yl)methyl)quinolin-8-ol as potential antiviral SARS-CoV-2 candidate: Synthesis, crystal structure, Hirshfeld surface analysis, DFT and molecular docking studies. J Mol Struct 1232: 130005.
[Crossref], [Google Scholar], [Indexed]
- Arulmani R, Sankaran KR (2014) Synthesis, spectral, SHG efficiency and computational studies of some newly synthesized unsymmetrical azines of 4 biphenyl carboxaldehyde. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 129: 491-498.
[Crossref], [Google Scholar], [Indexed]
- Ganga M, Sankaran KR (2020) Synthesis, spectral characterization, DFT, docking studies and cytotoxic evaluation of 1-(4-fluorobenzyl)-2, 4, 5-triphenyl-1H-imidazole derivatives. Chemical Data Collections 28: 100412.
[Crossref], [Google Scholar]
- Amala S (2019) The synthesis of 3-ethyl-5-methyl-2, 6-diarylpiperidin-4-on-1-ium picrates and their spectral, XRD and theoretical studies. New Journal of Chemistry 43(27): 11003-11014.
[Crossref], [Google Scholar]

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences