ISSN : 0976-8505
Der Chemica Sinica
Synthesis, Spectral Characterisation and MIC Evaluation of Schiff Base Ligands Derived from o-Vanillin
PG and Research Department of Chemistry, Seethalakshmi Ramaswami College, Tiruchirappalli-620 002, Tamil Nadu, India
Abstract
The synthesis, characterization and Antimicrobial screening of Schiff base ligands 2-methoxy -6-((p-tolylimino) methyl phenol (L1), 2-((2- hydroxyl-3- methoxybenzylidene) amino) benzoic acid (L[2]) and 3-methoxy-4-((3- hydroxyphenylimino) methyl) phenol(L3) were discussed in this paper. The ligands were synthesized and it was characterized by Analytical data, melting point, IR, UV and 1H-NMR. The MIC evaluations of antimicrobial activity of the Schiff bases have been investigated and were recorded.
Keywords:
Schiff base ligands, synthesis, IR, UV, MIC evaluation.
Introduction
In coordination chemistry a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex [1,2]. Coordination compounds are having great impact and importance in biological system [3].
Schiff bases are aldehyde or ketone like compounds in which the carbonyl group is replaced by an imine or azomethine group. They are widely used for industrial purposes and also exhibit a broad range of biological activities [4].
Schiff base ligands have wide range of biological properties [5,6] and they are used in the fields of antitumor [7], antiviral [8], antifungal [9], antibacterial [10] and anti-inflammatory [11]. As biological models, they help in understanding the structure of biomolecules and biological processes occurring in living organisms. They are involved in the treatment of cancer drug resistance, and often tested as antimalarial [12].
Schiff base ligands may contain variety of substituents with different electron-donating or electron-withdrawing groups, and therefore may have interesting chemical properties. They have attracted the scientists due to the particular interest due to their biological activities [13-16].
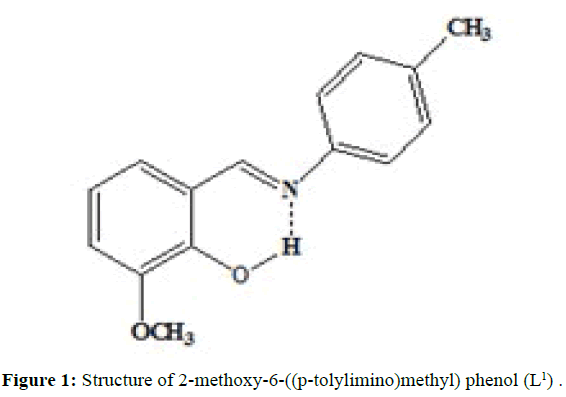
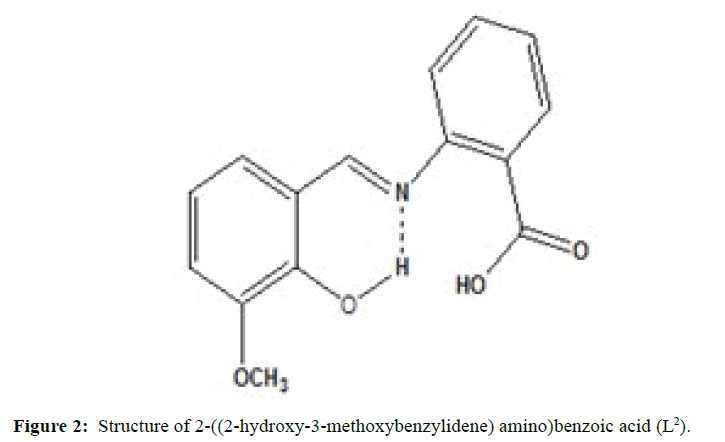
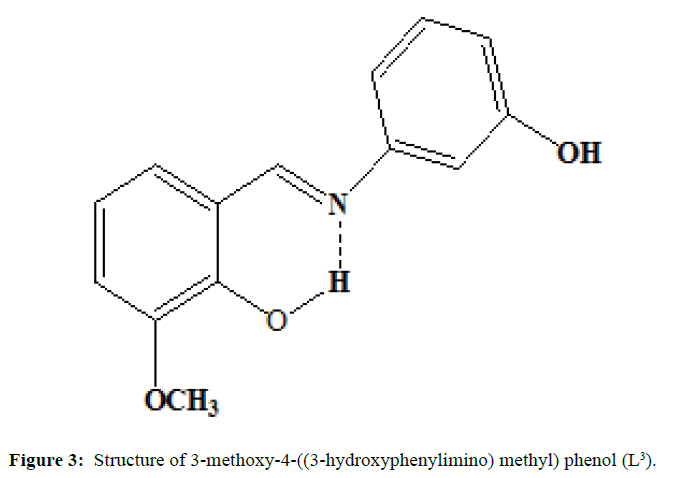
(Figures 1-3) In this study we have synthesized the ligands L1: 2-methoxy -6-((p-tolylimino) methyl phenol from o-vanillin and p-toluidine, L[2]: 2-((2- hydroxyl-3- methoxybenzylidene) amino) benzoic acid from o-vanillin and 2-amino benzoic acid, and L3: 3-methoxy-4-((3-hydroxyphenylimino) methyl) phenol from o-vanillin and 3- aminophenol. The ligands were synthesized and it was characterized by Analytical data, IR, UV and1H-NMR. The MIC evaluation of antimicrobial activity of the Schiff bases has been investigated.
Experimental
Materials and methods
The synthesisof ligand L[1] is done by, Equimolar quantity of o-vanillin (0.002 mol, 2.1342 g) and p-toluidine (0.002 mol, 3.0430 g) dissolved separately in ethanol and were mixed in RB flask and refluxed for 3 hours over a water bath. The mixture was cooled and orange coloured compound is separated, filtered, washed with small amount of alcohol and then with distilled water and dried. Melting point was noted. (M.P=91°C; Yield=80%)
Similarly the ligand L[2] has been synthesized by mixing the equimolarquantity of o-vanillin (0.01 mole, 1.52 g) and 2-aminobenzoic acid (0.01 moles, 1.367 g) were dissolved separately in ethanol. The o-vanillin solution was added drop wise. The above solution was magnetically stirred for about 1 h. The solvent was evaporated and cooled at room temperature, when brick red colored compound was separated out. It was washed with ether and recrystallized from ethanol. (M.P=99°C; yield=75%).
The synthesis of ligand L[3] is done by mixing of the equimolar quantity of o-vanillin (0.002 mole) and 3-aminophenol (0.002 mole) dissolved separately in ethanol are mixed in RB flask and heated under refluxed for 4 hours over a bath. The mixture is cooled and orange coloured compound is separated, filtered and washed with small amount of methanol and dried. Melting point was noted and purity was ascertained by TLC method (M.P=140°C; yield=84%)
The purity of the ligands checked by TLC method.
Results and Discussion
The characteristic and analytical data of Schiff base ligands were given in the (Table 1). The ligands are soluble in DMSO and DMF whereas insoluble in ether.
Vibrational spectra
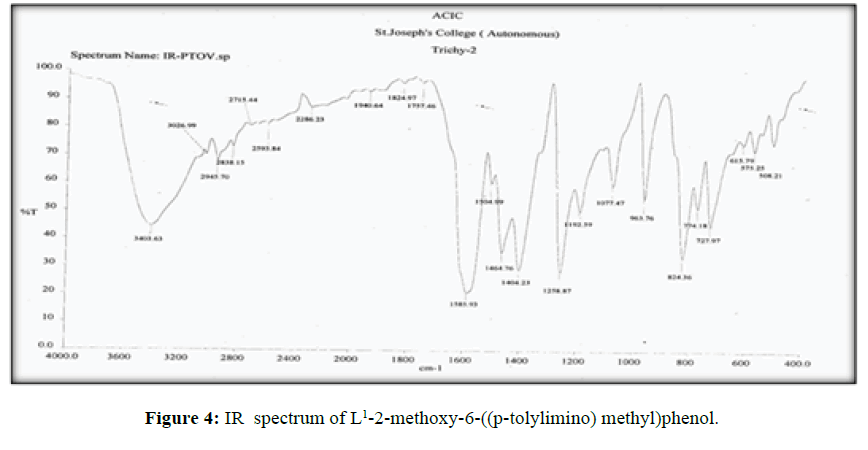
The vibrational spectra of the ligand (L[1]) (Figure 4) shows a band at 1585 cm-1 corresponds to ν (>C=N-) [14] and another broad band at 3403 cm-1 which is the characteristic frequency of hydrogen bonded ν (O-H).
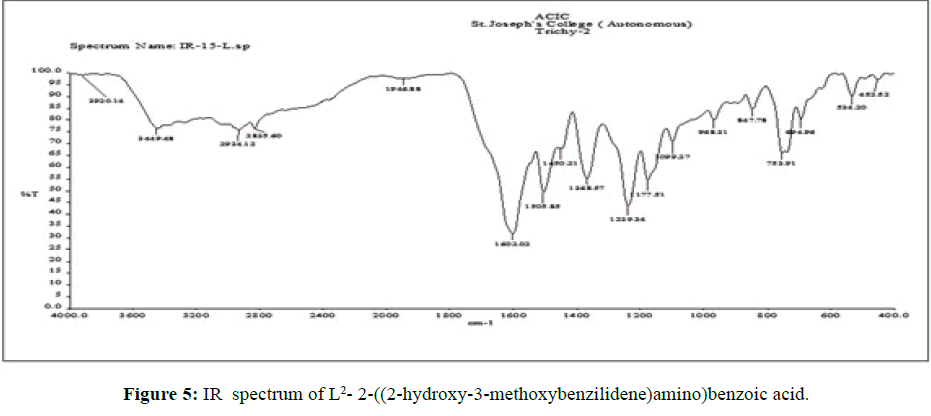
The vibrational spectra of the ligand (L[2]) (Figure 5) shows a strong band at 1602 cm-1 is assigned to the overlap of ν (C=O) and ν (>C=N-) stretching frequencies. The ν C–O (phenolic) stretching frequency of ligand is seen at 1450 cm-1 [15].
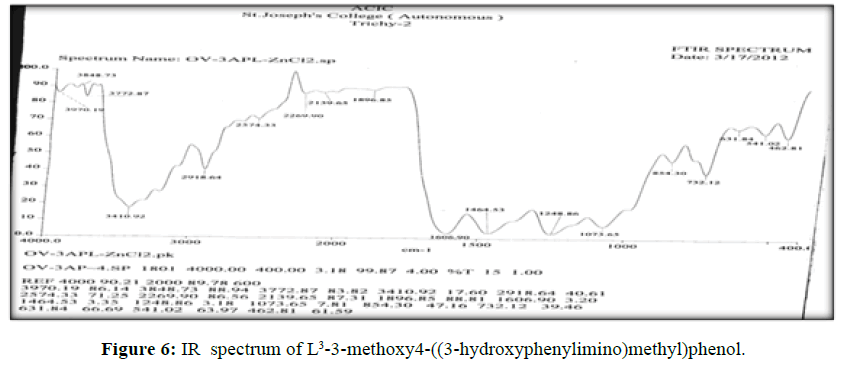
The vibrational spectra of the ligand (L[3]) (Figure 6) shows a band at 1603 cm-1 corresponds to ν (>C=N-) and ν (>C=O) (phenolic) displayed at 1460 cm-1. The broad band ranges from 3403- 3474 cm-1 which is the characteristic frequency of hydrogen bonded ν (O-H).
Electronic spectra
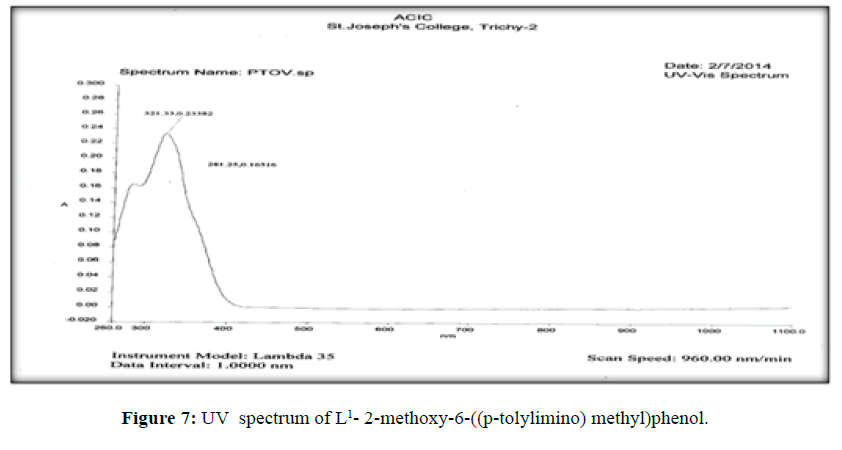
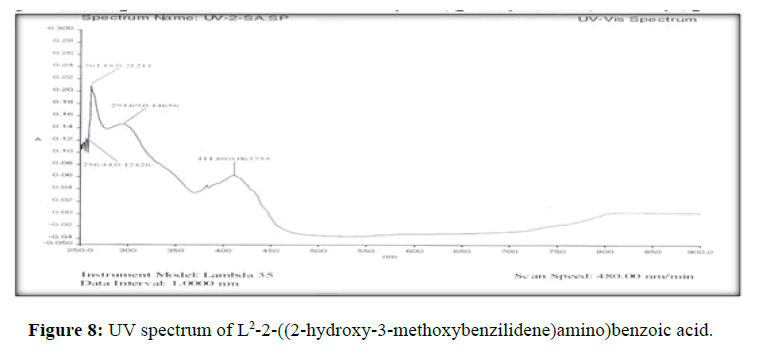
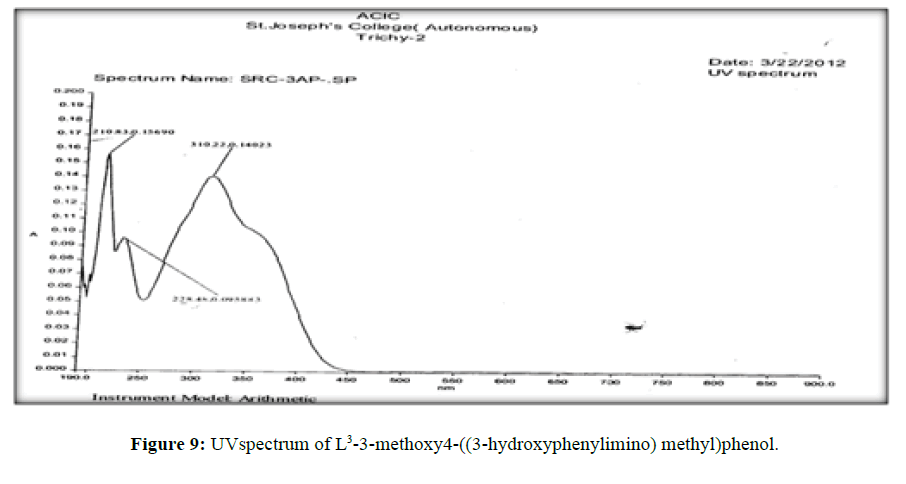
The electronic spectra of the ligands (L[1]) (Figure 7), (L[2]) (Figure 8) and (L[3]) (Figure 9) were recorded in DMSO. The spectrum of the ligand (L[1]) shows two absorption maxima at 321.22 nm (35587 cm-1) and 281.25 (31153 cm-1) which may be assigned to π-π* and n-σ* transition respectively. The spectrum of the ligand (L[2]) (Figure 5) shows three bands at 276 nm (36232 cm-1), 315 nm (31746 cm-1) and 356 nm (28090 cm-1). The band at 36232 cm-1 suggesting the presence of π-π* transition [16]. The spectrum of the ligand (L[3]) (Figure 6) is dark orange in colour shows 3 absorption bands at 210.38 nm, 228.48 nm, 310.22 nm at the frequency of 47431 cm-1, 43767 cm-1 and 32235 cm-1 could be assigned to π-π* and n-σ* transitions are due aromatic part of ligands of azomethine linkage in the Schiff Bases.
1H-NMR spectra
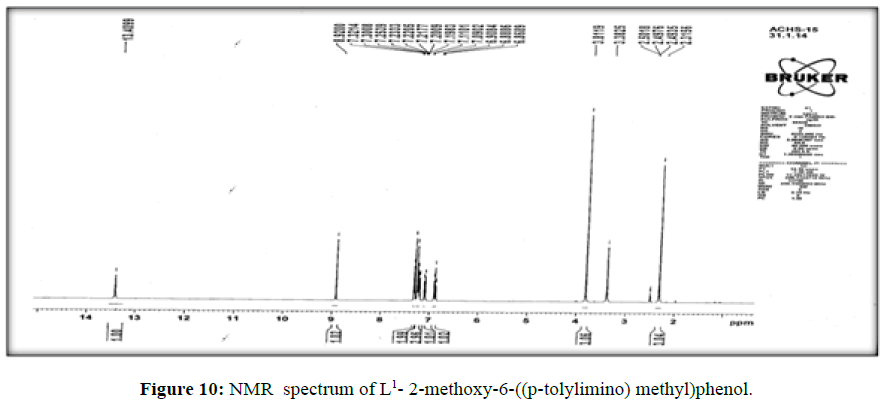
The 1H-NMR spectrum of the ligand (L[1]) (Figure 10) shows a signal at δ 8.9 ppm suggesting the presence of –CH=Nlinkage. The multiplet in the proton NMR spectrum which extends from δ 6.8 to 7.3 ppm corresponds to the seven protons of the aromatic ring [17].
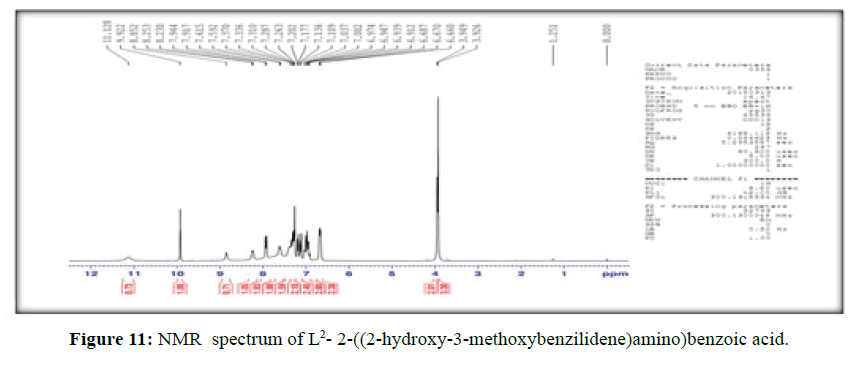
The 1H-NMR spectrum of the ligand, (L[2]) (Figure 11), shows a signal at δ 3.95 ppm and a strong signal at δ 11.13 ppm corresponding to –OCH3 and hydroxyl group of –COOH group respectively, the peak at δ 9.92 ppm is attributed to phenolic –OH group present in the ligand, the signal at δ 8.85 ppm is assigned to (-CH=N-) proton and the signals for the protons of the phenyl group attached to the ligand are observed in the range δ=6.67-7.94 corresponds to seven protons [18].
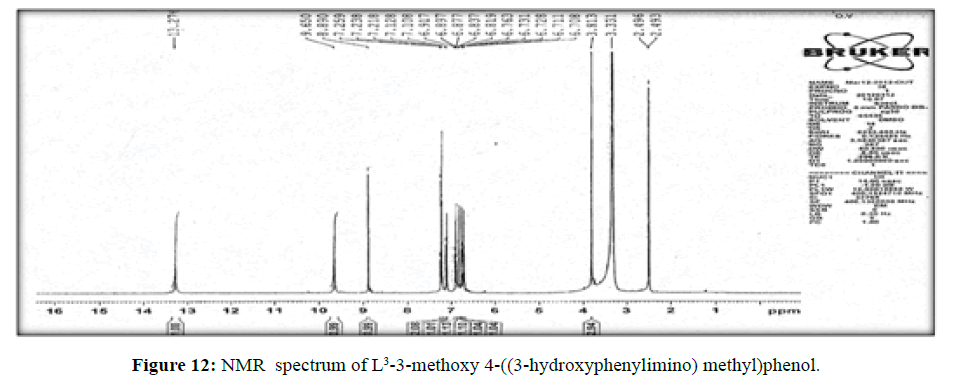
The spectrum of the ligand (L[3])(Figure 12) shows a signal at δ8.8 ppm a single peak suggesting the presence of – CH=N- linkage. The multiplet which extends from δ 6.7 to 7.3 ppm corresponds to the seven protons of the aromatic ring. The peak at δ 13.2 ppm indicates the hydroxyl proton.
Antifungal activities of the Schiff base ligands
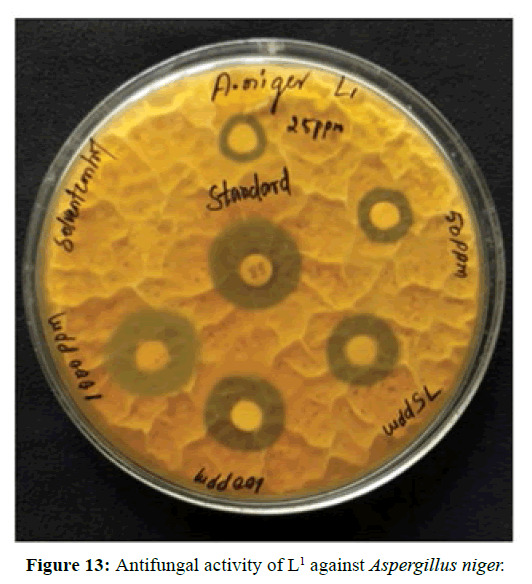
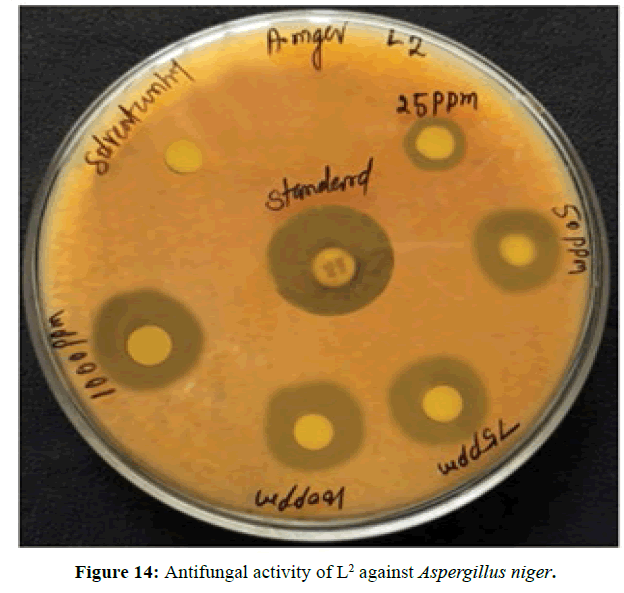
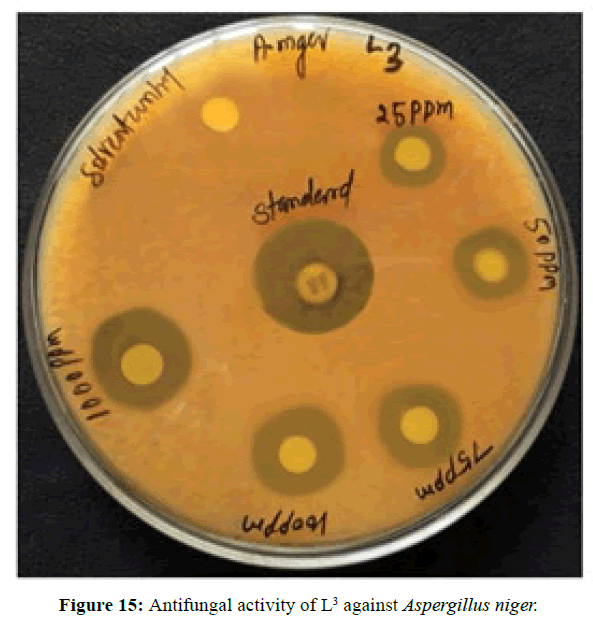
Antifungal activity of the Schiff bases has been tested by disc diffusion technique [19]. The fungi Aspergillus niger is used to find out the antifungal activity [20]. The results were compared with standard drug Nystatin for the fungus. The new ligands showed remarkable biological activities against the fungus. The data obtained after preliminary antifungal screening showed that the following ligands were most active against Aspergillus niger (Figures 13-15).
Minimum inhibitory concentration
The three synthesized Schiff base ligands exhibited varying degree of Inhibitory effects (low to moderate) on Aspergillus niger and are tabulated in (Table 2). Even at low concentration, 75 ppm, 2-((2-hydroxy-3- methoxybenzylidene) amino) benzoic acid (L[2]) and 4-((3-hydroxyphenylimino) methyl -3-Methoxyphenol (L[3]) are active, whereas 2-methoxy -6-((p-tolylimino) methyl phenol (L[1]) is moderately active at 100 ppm against Aspergillus niger.
Conclusion
Based on the above results the following conclusions are drawn, the ligands are acting as dibasic tridentate ligands. The melting points of the ligands are above 100°C, suggests that the three ligands are appreciably stable. After preliminary screening of synthesized ligands it is found that the three ligands are active against Aspergillus niger. So, Minimum Inhibitory Concentration was carried out. After the MIC evalution, it is found that the ligand L[2] has the highest activity than the other two ligands.
References
- Gary LM, Paul JF, Donald AT, Inorganic chemistry Prentice Hall. P, Pearson Education Inc, USA.
- Cleiton M da Silva, Daniel L da Silva, Luzia V Modolo, Rosemeire, Alves B, et al. (2011), Schiff bases: A short review of their antimicrobial activities, J Adv Res 2: 1-8.
- Phatak P, Jolly VS, Pathak P (1998) “Schiff base and their derivatives as potential anticancer agents, Ultra Sci Phys Sci 10: 263-266.
- Meng F, Zhao Q, Li M,.Xin Y, Yingyong H, (2003) Applications of metal complexes of schiff bases: A review. Chem Abstr 138: 330-746.
- Ramesh R, Sivagamasundari M (2003), Synthesis and antifungal activities of Ru(II) complexes, Synth React Inorg Met Org Chem 33: 1953-1963.
- Ranga SP, Sharma S, Chowdhary V, Parihar M, Mehta RK (1988) Spectral and antibacterial investigation on tridentate Schiff bases and their Co(II), Cu(II) and Ni(II) complexes, J Curr Bio Sci 5: 98-100.
- Kar DM, Sahu SK, Pradhan D, Dash GK, Misra PK (2003), Synthesis, antimicrobial and anti-inflammatory activities of some novel istinoid compounds, J Tech Res Chem10: 20-24.
- Rishu K, Harpreet K, Brij KK (2013), “Application of copper schiff bases: A review” Sci Revs Chem Commun 3, 1-15.
- Wang PH, Keck JG, Lien KJ, Lai MMC, Ear J (1990) Non- acidic anti-inflammatory compounds-activity of N-(4,6- dimethyl-2-pyridenyl-) benzamides and derivatives”, IJ Chem 25: 9.
- Tushar SB, Archana B, Asit KC, Xueqing S, George E et al. (2008), Synthesis, crystal structures, cytotoxicity and qualitative structure–activity relationship (QSAR) of cis-bis{5-[(E)-2-(aryl)-1-diazenyl]quinolinolato}di-n-butyltin(IV) complexes, nBu2Sn(L)2. J Inorg Biochem 102: 1719-1730.
- Abdel AWS, Hassan HY, Aboul-Fadl T (2010) Youssef AF Pharmacophoric model building for antitubercular activity of the individual Schiff bases of small combinatorial library. Eur J Med Chem 45:1098-1106.
- Morad FM, Ajaily M, Ben G S (2007) preparation spectroscopic characterization and antibacterial activity of new Schiff base complexes. Press J of Science and in Appl 1: 72-78.
- Maurya RC, Patel P, Rajput S (2003) Synthesis and characterization of mixed ligand complexes of Cu(II), Ni(II),Co(II), Zn(II) with a Schiff base derived from the sulfa drug sulphmerazine and 2.2’-bipyridine. Synth React Inorg Met Org Chem 33: 801-816.
- Soliman AA, Linert W (1999), Investigations on new transition metal chelates of the 3-methoxy-salicylidene-2-aminothiophenol Schiff base. Thermochim Acta 338: 67-75.
- Phaniband MA, Dhumwad SD (2007) Synthesis, characterisation and biological studies of Co(II), Ni(II), Cu(II) and Zn(II) complexes of Schiff base derived from 4-substituted carbostyrils. Trans Met Chem 32: 1117–1125.
- Valarmathy G, Subbalakshmi R (2014), Synthesis, spectral characterization, electrochemical and fluorescence studies of biologically active novel Schiff base complexes derived from E-4-(2-hydroxy-3-methoxy benzlideneamino)-N-(pyrimidin-2yl)benzenesulfonamide. Turk J Chem 38: 521-530.
- Simmons WW (1978), The saddler handbook of proton NMR spectra, Sadtler Research Laboratories Inc.Philadelphia,USA.
- Valarmathy G, Subbalakshmi R (2013), Synthesis, characterization and antimicrobial screening of Co(II), Ni(II), Cu(II),Mn(II) and Zn(II) complexes with Schiff base derived from 2-Sulphanilamidolpyridine and 2-hydroxy-3-methoxybenzaldehyde. Asian J Chem 25:2077-2079.
- Rahman, AU, Choudhary MI, ThomsenWJ (2001) Bioassay techniques for drug development, Harwood Academic Dordrecht.UK.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences