ISSN : 0976-8505
Der Chemica Sinica
Synthesis of Organoclay and its Applications in Electrochemical Detection of Paracetamol
Valery HambateGomdje1*, Abdoul Ntieche Rahman2, Abdoul Wahabou3, BenoîtLoura1 and Abdelilah Chtaini4
1The Higher Institute of the Sahel, University of Maroua, Cameroon
2Higher Teachers’ Training College, University of Maroua, Cameroon
3Institute of Mines and Petroleum Industries, University of Maroua, Cameroon
4Team of Molecular Electrochemistry and Inorganic Materials, Faculty of Sciences and Technology of Beni Mellal, University of Sultan Moulay Slimane, Morocco
Abstract
The aim of this work is to synthesize by cationic exchange reaction organoclays by exfoliation after modification of clay with cetyltrimethylammonium ions (CTA+) and their use as an electrode material for the detection of Paracetamol. The physicochemical properties of modified and unmodified clay mineral are ï¬ÂÂrst analyzed by particle size analysis, XRD, BET, FTIR, thermal analysis (TGA and DTA), 29Si NMR, 27Al NMR and TEM. The results showed that the surface of clay successfully reacted with cetyltrimethylammonium bromide (CTAB) and this signiï¬ÂÂcantly enhanced electrochemical reactivity. This electrochemical sensor exhibited excellent analytical performance for Paracetamol detection at physiological pH with detection limit of 23 μM, linear range of 10-80 μM (R2=0.9825).
Keywords
Cetyltrimethylammonium ions, Electrochemical sensor, Organoclays
Introduction
Modification of clay minerals surface has received attention because it allows the creation of new materials and new applications. The synthesis of organoclays has opened new perspectives with those composites that have interesting and diverse applications in materials science. Modified clays have varied applications such as in the agro-food industries, in the cosmetics, water and air, control agents, etc.
In electrocatalysis, the modified clays are used as catalytic support and also as modifying electrodes for the detection of certain pharmaceutical compounds thus opening the way to the development of the new types of electrochemical sensors. Furthermore, its applications in pharmacy, adsorbents, and ion exchangers are also reported in the literature [1,2]. These last applications are particularly useful for the development of electrochemical sensors [3,4].
In recent years there has been considerable progress in the field of electrodes modified by organoclays [5-10]. The organoclays can be synthesized by several routes including the postsynthesis grafting of functionalized organosilane onto the clay surface [11-14], the one-step preparation by the sol-gel process [15,16] and the intercalation of quaternary ammonium-based organic cations in the interlayer region of the clay [17-20]. The new chemical properties exhibited by the electrode surfaces when coated by the clays are indicative of the catalytic power of the modified clays. In the latter case (intercalation of quaternary ammonium cations), the novel properties are exploited for applications such as ion exchange coatings, or (electro)-chemical sensors [21,22].
The previous work has been based more on the formation of organoclays by reaction with silane groups [9]. For example, the treatment of montmorillonite with trichloro and trialkoxysilanes has been reported, resulting in organic loadings of up to 25 wt %, with the intended application of hazardous material remediation [23]. No increase in the clay’s basal spacing has been observed, suggesting that the organic compounds are bound to the outer clay edges. The trialkoxysilanes apparently linked the clay sheets together, making them non dispersible, while monoalkoxysilane treated clays are dispersible in water. In another report, protonated amino alkoxysilanes and end-terminated alkoxysilanes are used to create clay monoliths by cross-linking clay particles together with their edges [24]. Also, the surface of magadiite, a layered sodium polysilicate containing silanol groups, is reacted with different lengths of aliphatic alcohols and is able to be cast into transparent nanocomposite films [25]. Surfactant-treated montmorillonite was further modified with a trialkoxysilane-terminated epoxy to improve clay compatibility and properties of poly(Llactide) and poly(L-lactide)/poly(butylene succinate) blends [26-28].
Quaternary ammonium ions have been frequently used to prepare organoclays thus changing the hydrophilic properties of clays [29]. Falaras and Petridis have also reported the preparation of Clay-Modified Electrodes (CME) by the coating of cetyltrimethylammonium bromide, which are successfully used for the incorporation and binding of anionic species [30]. Recently, Heidarimoghadam et al. [31] reported the successful application of graphene oxide in the presence of cetyltrimethylammonium bromide to the determination of testosterone in biological fluids and drug. In the case of smectites, the adsorption of neutral molecules is due to various interactions. The presence of the hydroxyl functions on the surface of these clays therefore allows the grafting reactions [32]. The organoclays are generally prepared in solutions by cation exchange reaction or by solid-state reaction.
Paracetamol is a widely used antipyretic and analgesic drug [33]. It is an effective and safe analgesic agent used worldwide for the relief of mild to moderate pain associated with headaches, backaches, arthritis and post-operative pains. It is also used for reduction of fevers of bacterial or viral origin [34]. Paracetamol has no toxic effect on human health, but its misuse can cause adverse effects, excessive doses can cause skin rashes, liver disorders, nephrotoxicity and inflammation of pancreases [35]. Presence of Paracetamol has been extensively studied in human plasma and urine, but is also being studied in the aquatic environment with levels of up to 10 μg/L in natural water as reported by Kolpin [36]. Therefore the development of rapid and simple methods is a clinical requirement. Several techniques, namely liquid chromatography [37,38], electrophoresis [39,40], spectrophotometry [41] and electrochemical methods [42-44], are employed for the determination of drugs such as Paracetamol in pharmaceutical preparations. However, spectrophotometric and chromatographic methods usually require sample pretreatment (e.g., extraction, complex formation) that is laborious and time consuming. To overcome these defects, electrochemical techniques have received more attention in detection of Paracetamol due to the presence of electroactive groups of hydroxyl and acetamid in Paracetamol molecule. Conventional electrodes do not give a satisfactory response, which is why the development of new electrodes modified with organoclay composites emerged, opening the way to the promotion of electrochemical sensors [45-47].
In this work, the organoclays were synthesized by exchanged cationic reaction with cetyltrimethylammonium bromide. The characterization techniques such as analysis of particle size, XRD, BET, FTIR, TGA/DTA, 29 Si and 27 Al NMR and TEM were used to explain the physical and chemical properties of composites. The film layer of the organoclays was used to modify the surface of the glassy carbon electrode. The electrochemical behavior of the modified electrode was analyzed and its response to the electrochemical detection of Paracetamol was evaluated.
Materials and Methods
Materials
Paracetamol was purchased from Sigma-Aldrich. N-Cetyl-N,N,N-trimethyl ammonium bromide (C19H42BrN) Assay was obtained from Himedia Laboratories Pvt. Ltd, India. Sodium chloride (NaCl) was procured from Merck specialties Privates Limited Mumbai, India (≥ 99.0%). Potassium phosphate monobasic (99%), Sodium hydroxide and Hydrochloric acid were purchased from Sisco Research Laboratories Pvt. Ltd, India.
The soil samples were taken in a dry river bed situated between the center and Makabaye quarter of Maroua town (Cameroon) in the far-north region of Cameroon. The system coordinates of this area is 10°34.393N and 014°16.895 E. The chemical and mineralogical compositions of the clay were studied elsewhere [48]. The clay was purified according to the protocol described in the literature [49]. After these treatments, the clay slurry was centrifuged; settled clay was washed with distilled water (2-3 times) and dried at 100-110°C. Dried clay was ground using a mortar and pestle, and sieved to collect particles with less than 2 μm fraction. The purified clay is designated as Clay Ma. Its cation exchange capacity (CEC), 63 meq/100 g, was determined by NH4+ method as described in the literature [50].
Modification of Clay
Preparation of Organoclays
The preparation of organoclay complex was obtained by very slow addition of 500 mL of the corresponding CTAB solution at 0.5 CEC (initially homogenized by stirring for 1 h). At the end of this addition, which is under rapid and permanent agitation, the resulting mixture was allowed to stand for 24 h at room temperature to promote insertion of the cationic surfactant. After 24 h, the organoclay complex obtained was separated by centrifugation at 7000 rpm for 30 min. The pellet obtained was washed several times with deionized water until the disappearance of excess surfactant (no foam). After drying at 40°C in an oven, the material obtained was ground in a porcelain mortar. The solutions were decanted and suction ï¬ÂÂltered. The precipitate was washed with 100 mL of deionized water, stirred for 1 h, and suction ï¬ÂÂltered again. Washing and ï¬ÂÂltration were repeated up to 10 times to remove any residual CTAB. After each wash cycle the waste solutions were checked for Br ions (free CTAB molecules) by adding some drops of 0.5 M AgNO3. After the last ï¬ÂÂltration, the ï¬ÂÂltrate was dried in an oven at 80°C overnight, crushed to a ï¬ÂÂne powder in a mortar, and stored in a sealed container for further studies. This organoclay sample was labeled as CTAMa.
Preparation of the working electrode
The glassy carbon electrodes (GCE) were previously polished with alumina slurries of different size (1, then 0.05 μm) on billiard cloth. They were then placed in an acetone solution and properly cleaned in a sonicator for 10 min to eliminate any remaining alumina particles. The thin clay mineral ï¬ÂÂlm working electrode was prepared by “drop coating” 10 μl of the aqueous dispersion of CTAMa on the active surface (3 mm in diameter) of the GCE. The clay mineral modiï¬ÂÂed electrodes, denoted GCE/CTAMa, were stored at room temperature for about 6 h to ensure their complete drying before use.
Characterization techniques
Analysis of the particle size of sodium saturated clay and modified clay in aqueous solution was carried out at room temperature using Nanotrac equipped with Microtrac FLEX 10.5.2 software for data processing. The particle size distribution curves were obtained with organoclays samples prepared by the wet chemical method. For all the measurements, 0.0015 g each sample was sonicated in 20 ml millipore water about 10 minutes beforethe sample was subjected to particle size analysis.
The X-ray diffraction (XRD) patterns of the starting clay mineral and organoclays were recorded at room temperature using a Panalytical (Model: PW3040/60 X’pert PRO, Netherlands) equipped with a Cu anode (kα radiation, λ=1.54056 Å) and using a voltage of 40 kV and a current of 30 mA.
Speciï¬ÂÂc surface areas and pore volumes were evaluated by the B.E.T. method from N2 adsorption–desorption experiments performed at 77.35 K in the relative pressure range from 10-5 to 0.99, using an Autosorb iQ Station 2 (model Quantachrome Instruments). Prior to each measurement,the sample was degassed at 105°C under vacuum for 16 h.
IR spectra were scanned using KBr pellets in the region 4000 to 400 cm-1 with a resolution of 0.125 cm-1, on a spectrometer (model Tensor 27, Brucker Optik GmbH, Germany) equipped with the Opus TM software which provides an intuitive interface and facilitates the analysis of scans.
Differential thermal analysis (DTA) and thermogravimetric analysis (TGA) were recorded using a SDT Q600 V8.3 Build 101 simultaneous DSC-TGA instrument. Approximately 5.2600 mg of clay material was placed on the microbalance of the STA analyzer, which was purged with nitrogen gas. The measurements were recorded from room temperature to 1100°C under nitrogen flow (100 ml min-1) with a heating rate of 20°C/min. Data analysis was performed using Universal V4.7A TA software package.
Solid state NMR spectra were recorded using a BRUKER AVANCE DMX 400 MHz spectrometer operating at the frequencies of 104.229, 105.8 MHz for 27Al(I=5/2),23Na (I=3/2), quadrupolar nuclei and 79.460 MHz for 29Si (I=1/2), respectively.
Transmission electron micrographs of the functionalized clays were taken in FEI, the Netherlands (Model: Tecnai20) transmission electron microscope with an accelerating voltage of 200 keV. Ultrathin sections of bulk specimens (~100 nm thickness) were obtained at -85°C using an ultra-microtome ï¬ÂÂtted with a diamond knife.
Analytical measurements
Cyclic voltammetry (CV) on all prepared GCE/CTAMa as well as on an unmodified GCE was performed on CHI 6084C electrochemical analyzer (USA). A three-electrode measurement cell equipped with a GCE/CTAMa (working), an Ag/AgCl (saturated KCl) reference electrode and a Pt wire auxiliary electrode was used for all measurements. Phosphate Buffer Solution 0.1M (pH=7.4) was used as electrolyte solution.
Results and Discussion
Structural characterization of clays
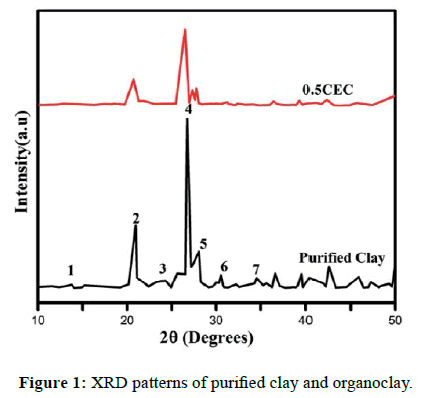
The X-ray diffraction of the purified clay (Figure 1) shows the presence of several characteristic peaks of each mineral. The natural clay of Makabaye is thus made up of illites, chlorites, quartz, kaolinite, Feldspar (Table 1). After modifying the clay with the surfactant (CTAB), the intensities of the peaks decreased, which could probably be due to the exfoliation of the interlayer layers. The approach of the intercalation is no more observable in this study because of the small amount of surfactant used which has been proven in previous work [51].
| Peak number | 2θ (°) | d-spacing (Å) | Minerals |
|---|---|---|---|
| 1 | 9.129 | 9.6790 | Illite (001) |
| 2 | 21.013 | 4.2243 | Quartz (100) |
| 3 | 25.635 | 3.4722 | Chlorite (004) |
| 4 | 26.955 | 3.3050 | Quartz (101)/ Illite (003) |
| 5 | 27.616 | 3.2275 | Kaolinite (002)/ Feldspar |
| 6 | 30.422 | 2.9359 | Illite |
| 7 | 34.422 | 2.5941 | Goethite |
Table 1: Reflections of purified clay
Porosity measurements
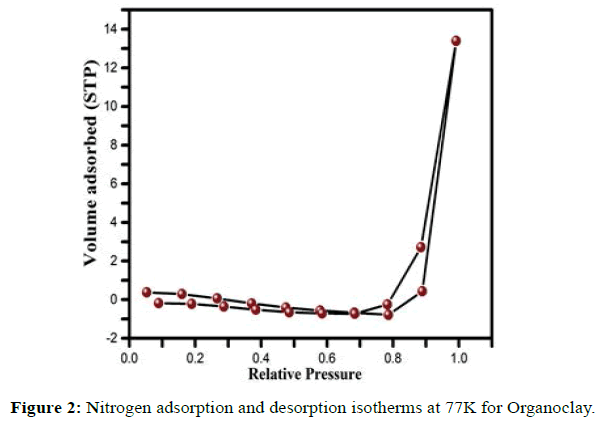
The adsorption-desorption isotherms for the organoclays are reported in Figure 2. They are typical of clay materials containing smectites [52]. The nitrogen adsorption-desorption isotherms for Organoclay are shown in Figure 2; the isotherms are type IV according to the I.U.P.A.C. classification and have an H1 hysteresis loop which is representative of mesopores [53]. The low value of the specific surface area (Table 2) is due to the presence of the organic molecules at the clay surface thus preventing the adsorption of nitrogen.
| Sample | Pore radius (Å) |
Specific surface area (m²/g) |
Pore volume (cm3/g) |
|---|---|---|---|
| CTAMa | 69.895 | 2.658 | 0.007 |
Table 2: Textural characterization of clays
Characterization of Purified Clay and Organoclay by FT-IR spectroscopy
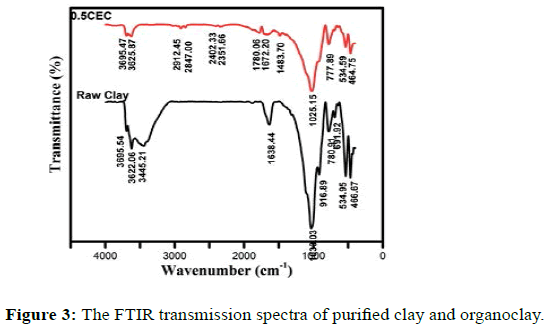
The FTIR spectra of the purified clay mineral and of organoclay are presented in Figure 3. They were found to coincide well with those reported in the literature for similar materials [54]. The two intense bands at 1025.15 and 1033.03 cm−1 were attributed to the Si–O stretching vibrations. The additional bands at 3695.54 and 3445.21 cm−1 due to hydroxyl stretching vibrations were attributed to free and interlayer water molecules, and 1638.44 cm−1 was related to the (H–O–H) bending vibrations of the water molecules adsorbed on the clay mineral. Other weak bands at 916.89 and 777.89 cm−1 were assigned to the bending vibrations of Al–Al–OH and Al–Mg–OH hydroxyl groups on the edges of the clay mineral layers [55]. The Si–O–Al (octahedral Al) and Si–O–Si bending vibrations were detected at 534.95 and 466.67 cm−1. After reacting with the CTAB solution, the organoclay exhibited new bands in the 2800-2950 cm−1 region, corresponding to antisymmetric and symmetric stretching of CH2 groups at 2912.45 and 2847 cm−1, respectively [56]. The characteristic band of the C-N stretching vibration (ν CN) of CTAB was present at 1471 cm−1 [57].
Thermal analysis
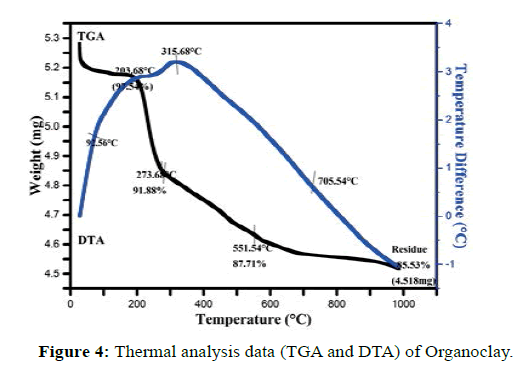
TGA and DTA thermograms of Organoclays are shown in Figure 4. The TGA curves indicate that there are three major steps during decomposition of the surfactant [58]. The corresponding TGA-DTA thermograms in the range of 25 to 1000°C revealed the loss of carbon from the organoclay in the 200-555°C region as indicated by three discrete “peaks” with mass-loss maxima at 203.68, 273.68, and 551.54°C. All these results are confirmed in DTA thermograms. The lowest temperature peak at 92.56°C is due to water loss from the surface (dehydration) and interlayers. The second at 315.68°C is due to degradation of CTAB in interlayers of clay, while the highest temperature peak at 705.54°C is due to dehydroxylation of the clay layers [59].
27Al and 29Si NMR Analysis
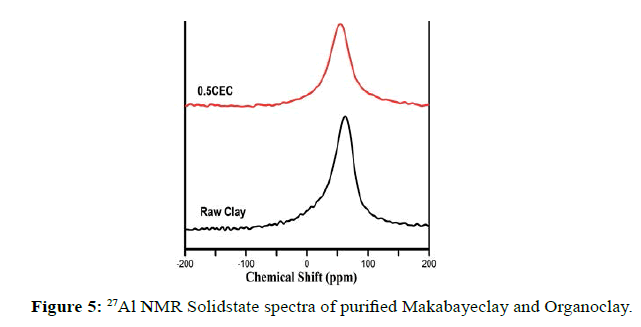
27Al and 29Si MAS NMR provides information about the inorganic framework. 27Al MASNMR spectra of Organoclay and Purified Clay samples are presented in Figure 5. In Purified Clay sample, one resonance line of the 27Al nuclei is observed at 66.64 ppm and one resonance line is observed at 55.25 ppm for Organoclay. This is related to tetrahedrally coordinated aluminum (IV) ions which occur in the octahedral layers of the clay. The interaction of tetrahedral layers with water molecules in the clay galleries was due to the generation of IV-coordinated aluminum ions in octahedrons [60,61].
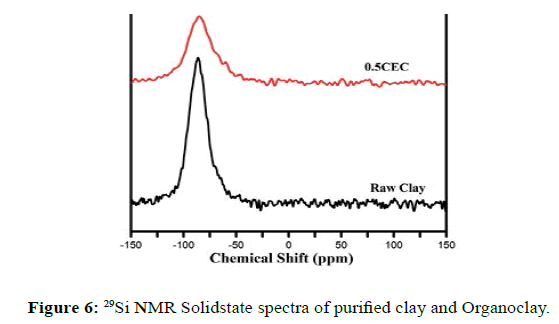
The 29Si MAS NMR spectra of purified clay (Figure 6) contained a single, symmetric 29Si resonance at -86.27 ppm [62], corresponding to the unique Si environment in the sample, consisting of Si atoms surrounded by three SiO4 tetrahedra-Q3(0Al) according to Liebau nomenclature [63]. The position of this signal does not change much with the treatment (-85.13 ppm), although an increase in the FWHM value was observed (Figure 6), indicating a progressive disorder in the local environment of the Si nuclei of the remnant montmorillonite particle [64-66].
Transmission Electronic Microscopy imaging of Organoclay morphology
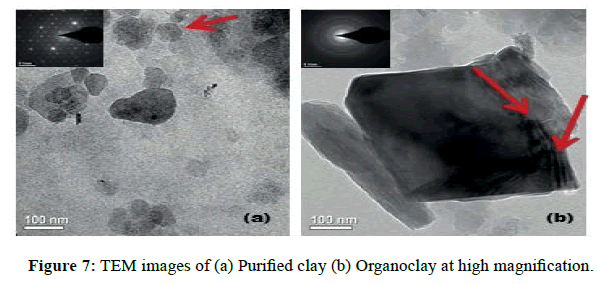
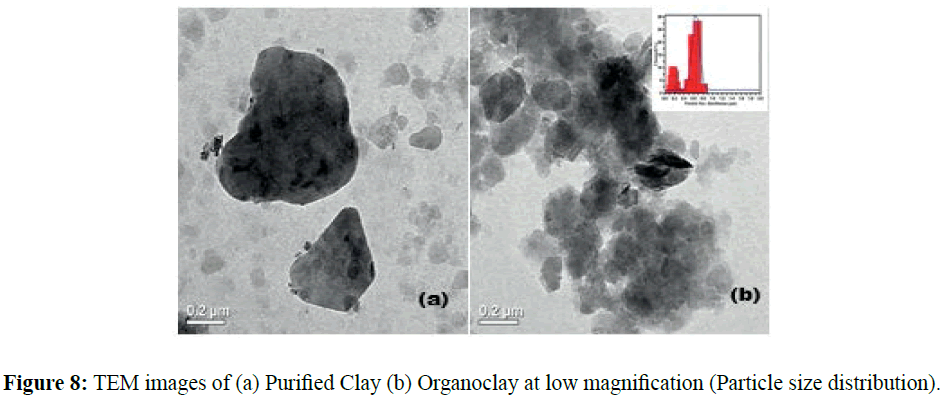
TEM images of Figures 7 and 8 shows the morphology of the Purified and Organoclay samples and seem identical. The presence of the interlayer silicates is observable with particles of hexagonal shape and dimensions of the order of 100 nanometers (Figures 7 and 8). The Organoclay particles have a bimodal distribution. The selected area electron diffraction shows the single crystallite diffraction for non-modified clay (Figure 7a) and the polycrystallite diffraction for Organoclays (Figure 8b).The image of Figure 7b shows the exfoliated layer.
Application
Electrochemical behavior of paracetamol at CTAMa modified electrode
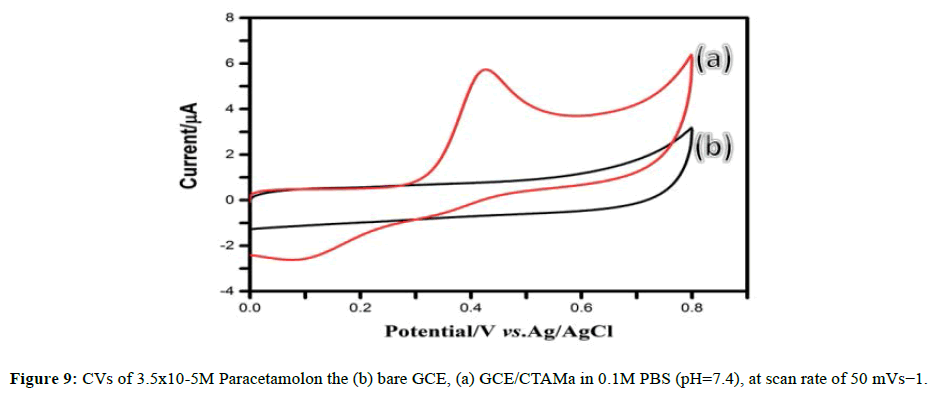
The voltammetric behavior of paracetamol on GCE/CTAMa was examined using Cyclic Voltammetry (CV) (Figure 9). One peak of oxidation at 415.3 mv and another peak of reduction at 128.9 mV, corresponding to quasi-reversible electrochemical reaction of paracetamol, can be seen. The remarkable voltammetric response of paracetamol on the GCE/CTAMa can be reasonably ascribed to the electrocatalytic activity of organoclays, which improves the adsorption efficiency and electrochemical reactivity of paracetamol. It is suggested that, owing to its high adsorptivity, CTAB effectively modifies the surface chemistry of clay sheets, which provides an efficient interface and microenvironment for the electrochemical reaction of paracetamol.
The redox mechanism of Paracetamol on GCE/CTMa
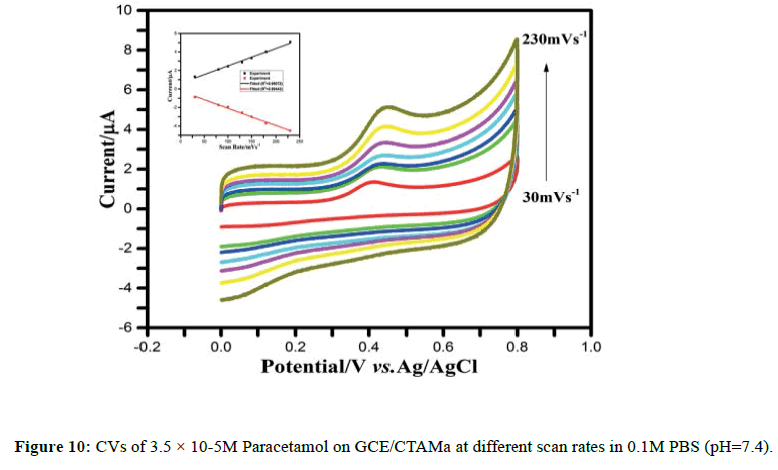
The effect of scan rate on the anodic and cathodic peak current of paracetamol on the GCE/CTAMa was investigated. As shown in Figure 10, the anodic and cathodic peak currents increase linearly as the scan rate grows from 30 to 230 mVs−1. The linear relationship between the peak current and the scan rate was obtained with the linear regression equation as: Ipa/μA=61.12+ 187.70 v/mVs−1 (R2=0.9907) and Ipa/μA=-22.12 -185.90 v/mVs−1 (R2=0.9944), respectively (Figure 10). This result indicates that the electrochemical reaction of paracetamol on the CTAMa ï¬ÂÂlm is a surface-controlled process [67].
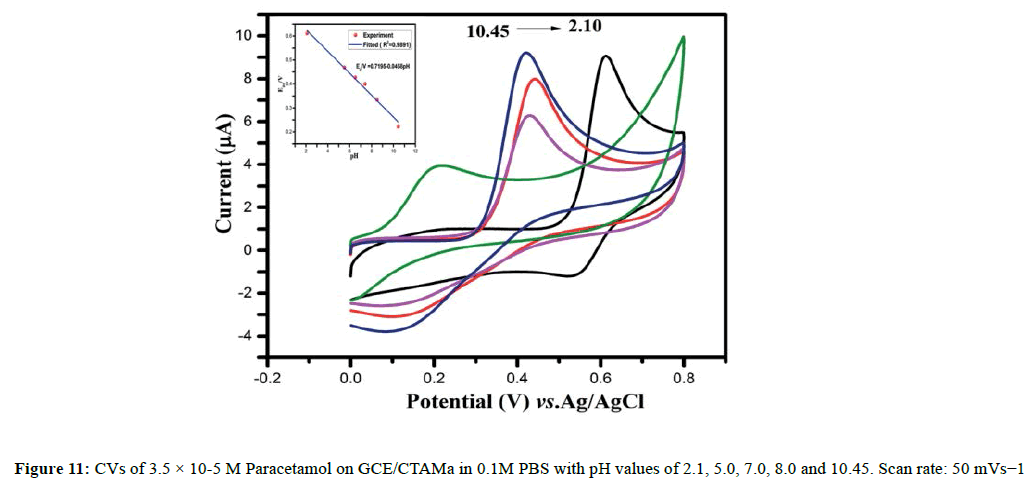
The effects of pH on oxidation of paracetamol on GCE /CTAMa have been studied in 0.1 M PBS in the pH range of 2.10-10.45 (Figure 11). With the increase of pH from 2.10 to 7.4, there has been significant increase in peak currents with an optimum pH at 7.4, followed by decline in peak currents from pH 7.4 to 10.45; the pH 7.4 is therefore chosen for the subsequent determinations. Furthermore, both oxidation peak potentials are shifted positively with increase in pH, confirming that protons participate in the oxidation processes of Paracetamol. The peak potentials are proportional to the pH values in the range of 2.10-10.45 (Figure 11) and the linear regression equations: EPa/V=0.7137-0.0484 pH (R2=0.9862).
According to the formula dEpa/dpH=2.303 mRT/nF
Where m is the number of protons; n, the number of electrons; and R, T and F have their conventional scientific meanings, the ratios of m/n were found to be 1.066 for paracetamol, suggesting the electrochemical oxidation of paracetamol two protons [68]. This is confirmed by the linear slopes of 48.49 mV/pH that are close to the theoretical value of 59 mV/pH.
Voltammetry determination of Paracetamol
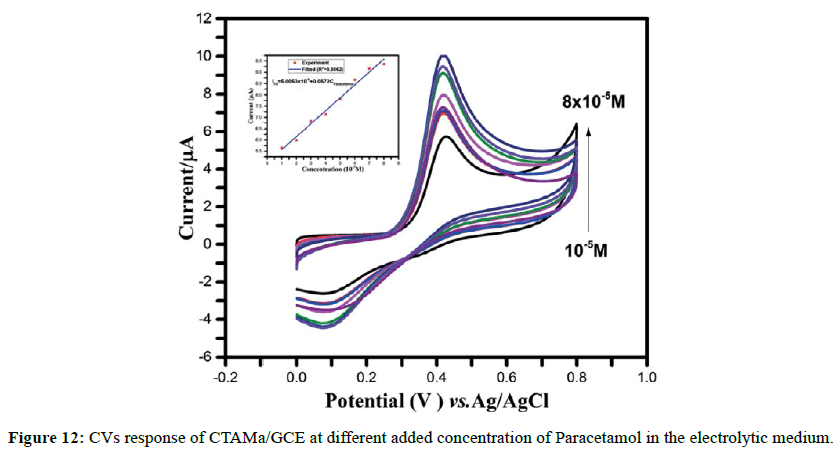
The voltammetric determination of paracetamol was carried out in 0.1 M PBS (pH 7.4) using cyclic voltammetry at the GCE/CTAMa (Figure 12). The oxidation peak current increases linearly with the concentration of paracetamol within the range of 10-80 μm. The equation of the straight line is Ipa (μA)=5.0035+57.2139Cparacetamol (R2=0.9862) (Figure 12). The detection limit is calculated by using the formula 3σ/b where σ is the standard deviation of the blank and b is the slope of the calibration curve. The detection limit of the modified electrode towards paracetamol is found to be at 23 μm.
Conclusion
In this work, organoclays with CTAB and natural clays were synthesized. Their physicochemical structure, morphology and thermal properties were explored using various experimental techniques. The results indicated the exfoliation after modification of natural clay with CTAB. It was found that the organoclays nanocomposite could provide a favorable interface and microenvironment for the electrochemical detection of paracetamol. The composite ï¬ÂÂlm modiï¬ÂÂed electrode was successfully employed for the voltammetric determination of paracetamol with low detection limit, wide linear range and good selectivity. The application of GCE/CTAMa for paracetamol detection in commercial tablets with satisfactory results was also demonstrated.
Acknowledgements
This work was financially supported by The World Academy of Sciences for the advancement of science in developing countries (TWAS) and the Council of Scientific and Industrial Research (CSIR). We thank also Dr. Vijayamohanan K Pillai, Director of Central Electrochemical Research Institute (CECRI) of Karaikudi, Tamilnadu (India) who gave us all the facilities to carry out our research. Thanks to Dr. A.K. Nanda Kumar who helped in the analysis and interpretation of SEM and TEM results.
References
- Vaccari A (1998) Preparation and catalytic properties of cationic and anionic clays. Catalysis Today 41: 53.
- Vaccari A (1999) Clays and catalysis: a promising future. Appl Clay Sci 14: 161.
- Mousty C (2004) Sensors and biosensors based on clay-modified electrodes-new trends. Appl Clay Sci 27: 159.
- Maghear A, Cristea C, Marian A, Marian IO, Sandulescu R (2013) Physico-chemical and electroanalytical characterization of two Romanian clays with possible applications in pharmaceutical analysis. Farmacia 61: 648.
- Mbouguen JK, Ngameni E, Walcarius A (2006) Organoclay-enzyme film electrodes. Anal Chim Acta 578: 145-155.
- Maghear A, Tertiş M, Fritea L, Marian IO, Indrea E, et al. (2014) Tetrabutylammonium-modified clay film electrodes: Characterization and application to the detection of metal ions. Talanta 125: 36-44.
- Kamga Wagheu J, Forano C, Besse-Hoggan P, Tonle IK, Ngameni E, et al. (2013) Electrochemical determination of mesotrione at organoclay modified glassy carbon electrodes. Talanta 103: 337-343.
- Yuan S, Chen W, Hu S (2004) Simultaneous determination of cadmium (II) and lead (II) with clay nanoparticles and anthraquinone complexly modified glassy carbon electrode. Talanta 64: 922-928.
- Tchinda AJ, Ngameni E, Tonle IK, Walcarius A (2009) One-Step Preparation of Thiol-Functionalized Porous Clay Heterostructures: Application to Hg(II) Binding and Characterization of Mass Transport. Chem Mater: 4111.
- Belkhamsa N, Ouattara L, Kawachi A, Tsujimura M, Isoda H, et al. (2015) Electrochemical analysis of Endocrine Disrupting chemicals over carbon electrode modified with Cameroon’s clay. J Electrochem Soc 16: B1-B8.
- Burkett SL, Press A, Mann S (1997) Synthesis, characterization, and reactivity of layered inorganic-organic nanocomposites based on 2:1 trioctahedral phyllosilicates. Chem Mater 9: 1071-1073.
- Celis R, Hermosin MC, Cornejo J (2000) Heavy Metal Adsorption by Functionalized Clays. Environ Sci Technol 34: 4593-4599.
- da Fonseca MG, Barone JS, Airoldi C (2000) Self-organized inorganic-organic hybrids induced by silylating agents with phyllosilicate-like structure and the influence of the adsorption of cations. Clays and Clay Miner 48: 638-647.
- Tonle IK, Ngameni E, Njopwouo D, Carteret C, Walcarius A (2003) Functionalization of natural smectite-type clays by grafting with organosilanes: physico-chemical characterization and application to mercury(II) uptake. Phys Chem Chem Phys 5: 4951-4961.
- Whilton N.T, Burkett S.L, Mann S (1998) Synthesis and Characterization of Allyl, Epoxy, Ethylenediamino and Imidazole-functionalized Magnesium Phyllosilicate Organoclays. J Mater Chem 8: 1927.
- Carrado K.A, Xu L, Csencsits R, Muntean J.V (2001) Use of organo- and alkoxy silanes in the synthesis of grafted and pristine clays. Chem Mater 13: 3766-3773.
- Ogawa M, Ishikawa A (1998) Controlled microstructures of amphiphilic cationic azobenzene montmorillonite intercalation compounds. J Mater Chem 8: 463-467.
- Li Z, Alessi D, Allen L (2002) Influence of quaternary ammonium on sorption of selected metal cations onto clinoptilolite zeolite. J Environ Qual 31: 1106-1114.
- Jiang J.Q, Cooper C, Ouki S (2002) Comparison of modified montmorillonite adsorbents: part I: preparation, characterization and phenol adsorption. Chemosphere 47: 711-716.
- Lee S.Y, Kim S.J (2002) Adsorption of naphthalene by HDTMA modified kaolinite and halloysite. Applied Clay Science 22: 55-63.
- Erdemoglu M, Erdemoglu S, Sayilkan F, Akarsu M, Sener S, et al. (2004) Organo-functional modified pyrophyllite: preparation, characterisation and Pb (II) ion adsorption property. Appl Clay Sci 27: 41-52.
- Colinson MM (2002) Recent Trends in analytical applications of organically modified silicates materials. Trends in analytical chemistry 21.
- Song K, Sandi G (2001) Characterization of Montmorillonite Surfaces after Organosilane Modification. C Clays and Clay Miner 49: 119-125.
- Bourlinos A, Jiang D.D, Giannelis E.P (2004) Clay-organosiloxane hybrids: a potential route to cross-linked clay particles and fabrication of clay monoliths. Chem Mater 16: 2404–2410.
- Mitamura Y, Komori Y, Hayashi S, Sugahara Y, Kurada K (2001) Interlamellar Esterification of H-Magadiite with Aliphatic Alcohol. Chem Mater 13: 3747–3753.
- Chen G.X, Yoon J.S (2005) Clay functionalization and organization for delamination of the silicate tactoids in poly (L-lactide) Matrix. Macromol Rapid Commun 26:899-904.
- Chen G.X, Kim H.S, Shim J.H, Yoon J. S (2005) Role of epoxy groups on clay surface in the improvement of morphology of Poly(L-lactide)/Clay composites. Macromolecules38: 3738-3744.
- Chen G.X, Kim H.S, Kim E.S, Yoon J.S (2005) Compatibilization-like effect of reactive organoclay on the poly(l-lactide)/poly(butylene succinate) blends. Polymer 46: 11829-11836.
- Metz S, Anderson R.L, Geatches D.L, Suter J.L, Lines R, et al. (2005) Understanding the swelling behavior of modified nanoclay filler particles in water and ethanol. J Phys Chem 119: 12625-12642.
- Falaras P, Petridis D (1992) Incorporation of anionic species in organoclay-modified electrodes. J Electroanal Chem 3: 229.
- Heidarimoghadam R, Akhavan O, Ghaderi E, Hashemi E, Mortazavi S.S, et al. (2015) Label free graphene oxide for rapid determination of testosterone in the presence of cetyltrimethylammonium bromide. Mat Sci Eng C: 61.
- Bergaya F, Lagaly G (2001) Surface modification of clay minerals. Applied Clay Science 19: 1-178.
- Boopathi M, Won M.S, Shim Y.B (2004) A sensor for acetaminophen in a blood medium using a Cu (II)-conducting polymer complex modified electrode. Analytica Chimica Acta 512: 191-197.
- De Carvalho R.M, Freire R.S, Rath S, Kubota L.T (2004) Effects of EDTA on Signal Stability During Electrochemical Detection of Acetaminophen. J Pharm Biomed Anal 34: 871-878.
- Li J, Liu J, Tan G, Jiang J, Peng S, et al. (2014) High-sensitivity paracetamol sensor based on Pd/graphene oxide nanocomposite as an enhanced electrochemical sensing platform. Biosens Bioelectron 54: 468-475.
- Nunes B, Antunes S, Santos J, Martins L, Castro B (2014) Toxic potential of paracetamol to freshwater organisms: A headache to environmental regulators? Ecotoxicol Environ Saf 107: 178-185.
- Gioia MG, Andreatta P, Boschetti S, Gatti R (2008) Development and validation of a liquid chromatographic method for the determination of ascorbic acid, dehydroascorbic acid and acetaminophen in pharmaceuticals. J Pharm Biomed Anal 48: 331-339.
- Carrera V, Sabater E, Vilanova E, Sogorb MA (2007) A simple and rapid HPLC-MS method for the simultaneous determination of epinephrine, norepinephrine, dopamine and 5-hydroxytryptamine: application to the secretion of bovine chromaffin cell cultures. J Chromatogr B Analyt Technol Biomed Life Sci 847: 88-94.
- He YF, Liu AL, Xia XH (2004) Poly(dimethylsiloxane) microchip capillary electrophoresis with electrochemical detection for rapid measurement of acetaminophen and its hydrolysate. Anal Bioanal Chem 379:1062-1067.
- Wei S, Song G, Li JM (2005) Separation and determination of norepinephrine, epinephrine and isoprinaline enantiomers by capillary electrophoresis in pharmaceutical formulation and human serum. J Chromatogr A 1098: 166-171.
- Khoshayand MR, Abdollahi H, Shariatpanahi M, Saadatfard A, Mohammadi A (2008) Simultaneous spectrophotometric determination of paracetamol, ibuprofen and caffeine in pharmaceuticals by chemometric methods. Spectrochim Acta A Mol Biomol Spectrosc 70: 491-499.
- Mazloum-Ardakani M, Beitollahi H, Sheikh Mohseni MA, Benvidi A, Naeimi H, et al. (2010) Simultaneous determination of epinephrine and acetaminophen concentrations using a novel carbon paste electrode prepared with , 2'-[, 2 butanediylbis (nitriloethylidyne)]-bis-hydroquinone and TiO2 nanoparticles. Colloids Surf B Biointerfaces 76: 82-87.
- Shahrokhian S, Ghalkhani M, Amini MK (2009) Application of carbon-paste electrode modified with iron phthalocyanine for voltammetric determination of epinephrine in the presence of ascorbic acid and uric acid. Sensors and Actuators B 137: 669-675.
- Mazloum-Ardakani M, Beitollahi H, Amini MK, Mirkhalaf F, Abdollahi-Alibeik M (2011) Simultaneous and selective voltammetric determination of epinephrine, acetaminophen and folic acid at a ZrO2 nanoparticles modified carbon paste electrode. Anal Methods 3: 673-677.
- Cheemalapati S, Palanisamy S, Mani V, Chen S (2013) Simultaneous electrochemical determination of dopamine and paracetamol on multiwalled carbon nanotubes/graphene oxide nanocomposite-modified glassy carbon electrode. Talanta 117: 297-304.
- Madrakian T, Haghshenas E, Afkhami A (2014) Simultaneous determination of tyrosine, acetaminophen and ascorbic acid using gold nanoparticles/multiwalled carbon nanotube/glassy carbon electrode by differential pulse voltammetric method. Sensors and Actuators B Chemical 193: 451-460.
- Zheng M, Gao F, Wang Q, Cai X, Jiang S, et al. (2013) Electrocatalytical oxidation and sensitive determination of acetaminophen on glassy carbon electrode modified with graphene–chitosan composite. Mater Sci Eng C Mater Biol Appl 33: 1514-1520.
- Tchangnwa FN, Haulin EH, Yanne E, Kabe C, Touogam BT, et al. (2015) Clay makabaye in the far north Cameroon: study chemical and mineralogical depth. Int J Bas App Sci 4: 109-115.
- Tributh H, Lagaly G (1986) Aufbereitung und Identifizierung von Boden- und Lagerstättentonen. GIT Fachz Lab 30: 524.
- He HP, Guo JG, Xie XD, Peng JL (2001) Location and migration of cations in Cu(2+)-adsorbed montmorillonite. Environ Int 26: 347-352.
- Ngassa GBP, Tonle IK, Walcarius A, Ngameni E (2014) One-step co-intercalation of cetyltrimethylammonium and thiourea in smectite and application of the organoclay to the sensitive electrochemical detection of Pb(II). Appl Clay Sci 99: 297-305.
- Bouwe RGB, Tonle IK, Letaief S, Ngameni E, Detellier C (2011) Structural characterization of ,10-orthophenanthroline-Montmorillonite intercalation compounds and application as low-cost electrochemical sensors for Pb(II) detection at the sub-nanomolar level. Appl Clay Sci 52: 258.
- Perez-quintanilla D, Sanchez A, del Hierro I, Fajardo M, Sierra I (2007) Preparation, characterization, and Zn2+ adsorption behavior of chemically modified MCM-41 with 5-mercapto-1-methyltetrazole. J Colloids Interf Sci 31: 551.
- Neaman A, Pelletier M, Villieras F (2003) The effects of exchanged cation, compression, heating and hydration on textural properties of bulk bentonite and its corresponding purified montmorillonite. Appl Clay Sci 22: 153-168.
- Ge P, Li F, Zhang B (2010) Synthesis of Modified Mesoporous Materials and Comparative Studies of Removal of Heavy Metal from Aqueous Solutions. Polish J Environ Stud 19: 301-308.
- Alexandre M, Dubois P (2000) Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Materials Science and Engineering: R: Reports 28: 1.
- Madejová J, Pentrák M, Pálková H, Komadel P (2009) Near infrared spectroscopy: a powerful tool in studies of acid-treated clay minerals. Vibrational Spectroscopy 49: 211-218.
- Zhu J, He H, Zhu L, Wen X, Deng F (2005) Characterization of organic phases in the interlayer of montmorillonite using FTIR and 13C NMR. J Colloid Interface Sci 286: 239-244.
- Khaorapapong N (2010) In situ complexation of thiourea in the interlayer space of copper(II)–montmorillonite. Appl Clay Sci 50: 414-417.
- Wang BX, Zhou M, Rozynek Z, Fossum JO (2009) Electrorheological properties of organically modified nanolayered laponite: influence of intercalation, adsorption and wettability. J Mater Chem 19: 1816-1828.
- Carrado KA (2000) Synthetic organo-and polymer–clays: preparation, characterization, and materials application. Appl Clay Sci 17: 1-23.
- Ohkubo T, Kennehashi K, Saito K, Ikeda Y (2003) Observation of two 4-coordinated Al sites in montmorillonite using high magnetic field strength 27Al MQMAS NMR. Clays and Clay Miner 51: 513-518.
- Takahashi T, Ohkubo T, Suzuki K, Ikeda Y (2007) High resolution solid-state NMR studies on dissolution and alteration of Na-montmorillonite under highly alkaline conditions. Microporous Mesoporous Mater 106: 284-297.
- Kinsey RA, Kirkpatrick RJ, Hower J, Smith KA, Oldfield E (1985) High-resolution Al-27 and Si-29 Nuclear Magnetic-Resonance spectroscopic study of layer silicates, including clay-minerals. American Mineralogist 70: 537-548.
- Liebau F (1985) Structural Chemistry of Silicates. Springer-Verlag, Berlin.
- Mantovani M, Escudero A, Becerro AI (2009) Application of 29Si and 27Al MAS NMR spectroscopy to the study of the reaction mechanism of kaolinite to illite/muscovite. Clays and Clay Miner 57: 302-310.
- Fan Y, Liu JH, Lu HT, Zhang Q (2011) Electrochemical behavior and voltammetric determination of paracetamol on Nafion/TiO2-graphene modified glassy carbon electrode. Colloids Surf B Biointerfaces 85: 289-292.
- Okoth OK, Yan K, Liu L, Zhang J (2016) Simultaneous Electrochemical Determination of Paracetamol and Diclofenac Based on Poly(diallyldimethylammonium chloride) Functionalized Graphene. Electroanalysis 28: 76-82.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences