ISSN : 2393-8854
Global Journal of Research and Review
Synthesis, Characterization and Electrochemical Study of Bioactive Ligand, 4-Hydroxy-3-Nitroacetophenone
1Dr. T. R. Ingle Research Laboratory, Department of Chemistry, S. P. College, Pune, India
2Department of Chemistry University of Pune, Pune, Maharashtra, India
Abstract
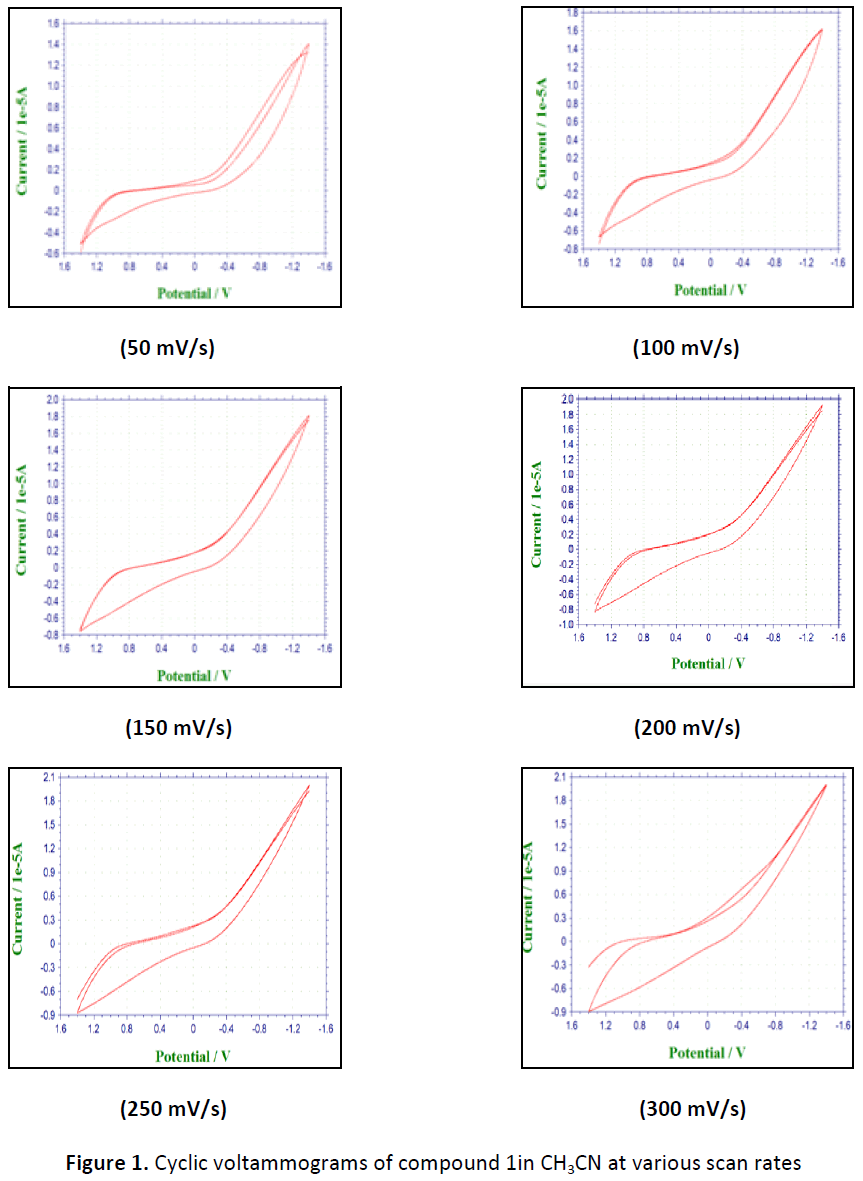
Cyclic voltammetry provides qualitative and quantitative information about the number of oxidation states and their stability, as well as the rate of heterogeneous electron transfer reactions. Variation of scan rate is an important tool of mechanistic investigations using cyclic voltammetry. The electrochemical behavior of the ligand is studied in an aprotic solvent, CH3CN. The ligand, 4 - hydroxy – 3 - nitroacetophenone, compound 1, has the antioxidant potential and it is expected that the metal complex increases its activity. Cyclic voltammograms of compound 1 proves cathodic peak potential at +0.95 V and anodic peak potential at -0.3 V at 100mV/sec scan rate. As ΔEp is greater than 0.059/n it is a quasi-reversible electron transfer with electron transfer rate const Ko = 2.8 x 10-8 cm/s. The results illustrate the response of ligand is quasi reversible in an aprotic solvent. The heterogeneous rate constant of electron transfer is calculated for the first time in an aprotic solvent.
Keywords
Cyclic voltammetry, Antioxidant potential, Quasi reversible, 4-hydroxy-3- nitroacetophenone.
INTRODUCTION
Cyclic voltammetry (CV) is the most commonly used method in electro catalysis. The technique offers a first view on electrode/electrolyte systems in the potential window of interest. For most metals and alloys, reproducible results can be obtained after cycling the surface in a base electrolyte and over suitable potential ranges. Cyclic voltammograms of some metal/electrolyte interfaces and some selected charge transfer reactions, of interest in fuel cell electro catalysis, have been reported. The use of CV for investigations, e.g., with ultra microelectrodes, rotating electrodes (disc and ring/disc), or in combination with online mass spectroscopy and FTIR spectroscopy, is outlined [1]. CV providing information not only on the thermodynamics of redox processes but also on the kinetics of heterogeneous electron-transfer reactions and coupled chemical reactions [2]. The characteristic shapes of the voltammetric waves and their unequivocal position on the potential scale, virtually fingerprint the individual electrochemical properties of redox systems [3]. The CV waves are corrected for solvent background employing Guttmann’scorrelation [4]. These experiments demonstrate the determination of the formal reduction potential (λ), the number of electrons transferred in redox process (n), the diffusion coefficient (D), electrochemical reversibility and the effects of varying concentrations and scan rates [5]. In CV the reversible heterogeneous charge transfer reaction showing ideal Nernstian reversible mechanism [6,7]. For reversible wave in SEV (Stationary electrode voltammograms) EP is independent of scan rate (v) and peak current, ip is proportional to v1/2 where the peak current is defined by Randles [8] and Sevcik [9]. The quasi-reversible systems were first exampled by Matsuda and Ayabe [10] in which ip may not be proportional to v1/2 and peak parameters ip,Ep,Ep/2 are functions of α , the charge transfer coefficient. The CV is characterized by several important parameters such as cathodic (Epc) and anodic (Epa) peak potentials, the cathodic (ipc) and anodic (ipa) peak currents, the cathodic half peak potential (Ep/2) and half-wave potential (E1/2). Rate constants for reversible quasi reversible and irreversible electron transfer reactions can be calculated.
MATERIALS AND METHODS
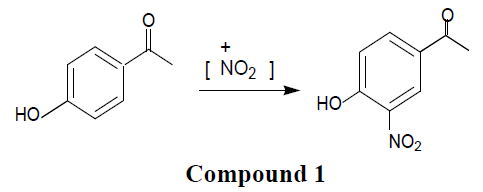
Reagents and solvents used were of commercially available analytical grade. compound 1 was synthesized by nitration of para-hydroxy acetophenone. This reaction mixture was refluxed in oil bath at 50°C for five minutes. Yellow solid with gummy mass poured into boiling water. Fine yellow product was obtained. The product was recrystallized from mixture of ethyl acetate and hexane. Sharp melting nature indicated purity. Formation of product was confirmed by reported values. Structure was confirmed by modern analytical spectral techniques. Cyclic voltammograms of the compound 1 was recorded in acetonitrile solution, used as solvent at 300K on ‘CHH Instrument Element Analyzer’ which is composed of three – electrode cell. Here internal standard comprises as a reference electrode Ag/AgCl and auxiliary Pt with working Pt electrodes. Electrochemical behavior of compound 1 was studied in an aprotic solvent acetonitrile at room temperature. Voltammograms of acetonitrile were recorded to eliminate the effect of solvent.
RESULTS AND DISCUSSIONS
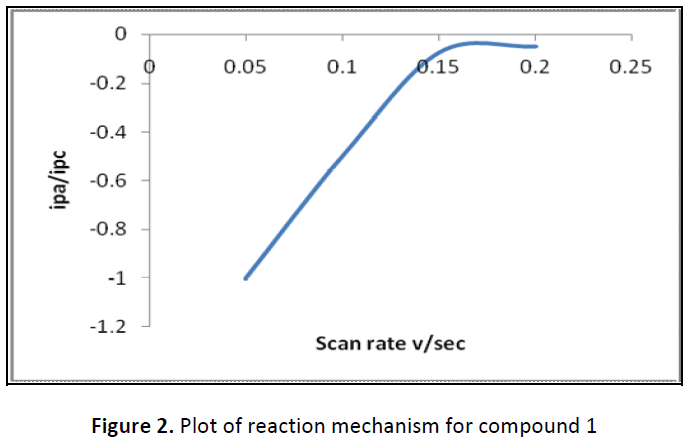
Cyclic voltammograms of compound 1, 4-hydroxy-3-nitroacetophenone proves cathodic peak potential at +0.95 V and anodic peak potential at -0.3 V at 100mV/sec scan rate. As ΔEp is greater than 0.059/n it is a quasi-reversible electron transfer with electron transfer rate const Ko = 2.8 x 10-8 cm/s and Ψp, current function. The plot of Ipa/Ipc Vs scan rate (Fig. 2) variation shows C type of mechanism, Ercir means reversible electron transfer followed by irreversible chemical reaction .The results have been reported (Table 1-4, Fig. 1).
Table 1: Electrochemical parameters with scan variation of (compound 1) in aprotic solvent (CH3CN)
| S. No. | Scan rate (Volts/Sec) | Epc (Volts) | Epa (Volts) |
|---|---|---|---|
| 1 | 0.05 | 0.95 | - 0.35 |
| 2 | 0.10 | 0.95 | - 0.30 |
| 3 | 0.15 | 0.95 | - 0.25 |
| 4 | 0.20 | 0.95 | - 0.20 |
| 5 | 0.25 | 0.95 | -0.20 |
| 6 | 0.30 | 0.85 | - 0.20 |
Table 2: Electro chemical data for quasi reversible charge transfer with scan rate variations of compound1 in aprotic solvent (CH3CN)
| ν | ν1/2 | Ipa | I pc | Ipa/Ipc | Ipc/ν1/2 | λ | Epc | Epa | ΔEp | E1/2 | τ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (v/sec) | (A) | (A) | (V) | (V) | (V) | (V) | (V) | (Sec) | |||
| 0.05 | 0.22 | 0.02 | -0.02 | -1.00 | 0.11 | -1.35 | 0.95 | -0.35 | -1.30 | 0.30 | 33.00 |
| 0.10 | 0.31 | 0.02 | -0.05 | -0.50 | 0.15 | -1.40 | 0.95 | -0.30 | -1.25 | 0.32 | 17.25 |
| 0.15 | 0.38 | 0.00 | -0.07 | -0.07 | -0.19 | -1.40 | 0.95 | -0.25 | -1.20 | 0.35 | 11.66 |
| 0.20 | 0.44 | 0.00 | -0.05 | -0.05 | -0.11 | -1.40 | 0.95 | -0.25 | -1.20 | -0.85 | 7.00 |
| 0.25 | 0.50 | 0.00 | -0.05 | -0.05 | -0.10 | -1.40 | 0.95 | -0.20 | -1.15 | -0.77 | 5.66 |
| 0.3 | 0.54 | 0.02 | -0.02 | 0.00 | -0.04 | -1.40 | 0.85 | -0.20 | -1.05 | -0.72 | 4.66 |
Table 3: Electrochemical and kinetic data for compound 1 in CH3CN
| V (v/s) | ipc (A) | Epc (v) | Epa (v) | ΔEp (v) | α na | Dx10-10 (cm2/s) | Kox10-8 (cm/s) |
|---|---|---|---|---|---|---|---|
| 0.05 | -0.02 | 0.95 | -0.35 | -1.30 | 0.04 | 0.02 | 1.56 |
| 0.10 | -0.05 | 0.95 | -0.30 | -1.25 | 0.05 | 0.04 | 3.13 |
| 0.15 | -0.07 | 0.95 | -0.25 | -1.20 | 0.05 | 0.06 | 4.73 |
| 0.20 | -0.05 | 0.95 | -0.20 | -1.15 | 0.05 | 0.02 | 3.13 |
| 0.25 | -0.05 | 0.95 | -0.20 | -1.15 | 0.05 | 0.01 | 3.13 |
| 0.30 | -0.02 | 0.85 | -0.20 | -1.05 | 0.06 | 0.03 | 1.58 |
Average rate constant (Ko) = 2.8745 x 10-8 cm/s, Co = 0.001 M A = 0.2826 cm2, Quasireversible charge transfer
Table 4: Type of reaction mechanism and redox potential (compound 1)
| Compound | Type of mechanism | Rate constant (Ko) cm/sec |
|---|---|---|
| 1 | Ercir | 2.8 x 10-8 cm/s |
| Er: O + e → R ← |
||
| Cir: R → Z |
CONCLUSION
Compound 1 undergoes electron transfer reaction in an aprotic solvent. It is synthesized for their electrochemical studies. The results indicate the stability of compound 1, a quasi-reversible reaction with rate const Ko = 2.8 x 10-8 cm/s and Epc value 0.95v .These are the important clues for their biological activity evaluation.
ACKNOWLEDGEMENT
Authors are thankful to the Principal and the Head, Department of chemistry, S.P. College, Pune for providing the necessary laboratory facilities for the work. Authors are also thankful to Department of Chemistry University of Pune for Instrumental facility.
REFERENCES
- Vielstich W., Handbook of Fuel Cells Published Online: 15 DEC 2010.
- Andrienko D., Cyclic Voltammetry, 22 Jan. 2008.
- Heinze J., Cyclic Voltammetry- “Electrochemical Spectroscopy”. New Analytical Methods (25), Priv.-Doz. Angewandte Chemie International Edition in English, Volume November 1984: 831–847.
- Ko H. H., Tsao L. T., Yu K. L., Liu C. T., Wang J. P., Lin C. N., Structure–activity relationship studies on chalcone derivatives: the potent inhibition of chemical mediators release, Bioorg. Med. Chem., 11(1), 2003: 105-111.
- Van Benschoten J.J., Cyclic voltammetry experiments, J. of Chem Edu., 60, 1983: 772.
- Kissinger P.K., Heineman, W.R. ‘Laboratory techniques in Electro analytical Chemistry’, Marcel Dekker, Inc. N.Y., 1984.
- Faulkner L.R. , Bard A. J., ‘Electrochemical methods, Fundamentals and Applications’, John Wiley and Sons Inc. 1980.
- Randles, J.E. B. Trans Faraday Soc., 44, 1948:327.
- Sevcik A., Collect. Czech. Chem. Commun., 13, 1948:349.
- Matsuda H. Ayabe Y., Elektrochem, 59, 1995:494.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences