ISSN : 0976-8505
Der Chemica Sinica
Synthesis, Characterization and Antimicrobial Activity Studies of New Amide Derivatives from Benzoyl derivatives of Amino Acid and Selected Sulfa Drugs by Using DCCI
Bushra K AL-Salami*
University of Basrah, Collage of Science, Department of Chemistry, Basrah, Iraq
Abstract
Several compounds which derived from condensation of N-benzoyl-DL-alanine, N-benzoyl glycine, N-benzoyl-Dvaline and N-benzoyl-DL-isoleucine with selected sulfa drugs such as sulfamethoxazole, sulfanilamide, sulfamerazine, sulfamethazine and sulfapyridine by using N,N-dicyclohexyl carbodiimide (DCCI) as an enhancer for the condensation process were synthesis. These compounds that can be considered as amides have been characterized by elemental analysis (C,H,N,S), FT-IR and 1HNMR. The synthesized compounds contain sulfa drug moiety shows excellent antimicrobial activity by using two kinds of bacteria, gram positive Staphylococcus aureus (S. aureus) and gram negative Aeromonas hydrophilic (A. hydrophilia) in different concentration and minimum inhibitory concentration MIC was founded.
Keywords:
N-benzoyl amino acid, Sulfa drug, DCCI, Condensation, Antibacterial activity.
Introduction
The current study focuses on the importance of preparing some amino acid derivatives like N-benzoyl amino acid and the reaction of these derivatives with some sulfa drugs to obtain new organic compounds which can afford some of the properties of the amino acids and sulfa drugs. Amino acids are organic compounds that are naturally involved in the synthesis of enzymes and peptides as well as proteins because these compounds have an acidic group (-COOH), which are carboxylic acid and amino group (-NH2). The amino acids show reactions of amine as well as carboxylic acid groups. In some cases, the amino group of these amino acids reacts with acid chlorides to form acyl derivatives such as N-benzoyl amino acid. Phthaloyl, phenyl methoxy carbonyl group (cbz), tert-butoxy carbonyl group (BOC) and N-benzoyl chloride which can be considered derivatives of amino acids.
Gramicidin and penicillin have been shown to be derivatives of D-amino acid [1]. Also, many complexes which prepared from amino acid have been reported in the literature for a long time [2-5]. Large number chemotherapeutically significant sulfa drugs such as sulfanilamide, sulfamerazine, sulfamethoxazole and sulfapyridine have been used as antibacterial agents which can be used to treat many diseases caused by some types of bacterial such as treatment urinary tract infections, throat and gum infections, eye infections and malaria [6,7]. Amide of sulfonic acids are called sulfonamides. The sulfa drugs are a class of sulfonamides. An amide is composite of a carboxylic acid and ammonia or its derivatives. Amides are least reactive of all acid derivatives because of delocalization which reduces the electrophilicity of carbon (of CONH2) [8]. The formation of amides from amine and carboxylic acid can be accelerated by using N,N-Dicyclohexyl carbodiimide. DCCI, which activates the carboxylic carbon of the amino acid derivatives so that the nucleophilic attack of amino group of sulfa drugs occurs readily at carbon. DCCI acts as dehydrating agent [9]. Aim of this investigation is to synthesize new amide which derived from N-benzoyl amino acids and selected sulfa drugs at room temperature by using the reagent DCCI. This reagent is the anhydride of a disubstituted urea, and when treated with water, it is converted to N,N1-dicyclohexyl urea (DCU) [10]. The reaction is efficient, and yields are generally very high.
Experimental
Materials and methods
The solvents, benzoyl chloride, glycine, DL-Alanine, DL-Valine and DL-Isoleucine were supplied by Merck. Sulfamethoxazole, sulfanilamide, sulfamerazine, sulfamethazine and sulfapyridine were acquired from sigma-Aldrich product. Additionally, all solvent used in particularly this work were of analytical grade. All measurements of melting point were done on a Bauchi 510.
Instruments: The spectra of Infrared (IR) were recorded as solid pellets by using potassium bromide on Shimadzu FT-IR spectrophotometer model 8400s in range 4000-400 cm-1. The spectra of 1HNMR were registered in a Bruker spectrophotometer (400 MHz) using DMSO-d6 as solvent and TMS as internal standard. Elemental analysis (C,H,N,S) were evaluated by Euro vector model 3000 A (Italy).
Synthesis of the compounds: Genral procedure for synthesis of N-benzoyl amino acids
N-benzoyl amino acids are most favorable prepared by the influence of benzoyl chloride upon amino acids in aqueous solution in the existence of some alkali, such as sodium hydroxide, is used to neutralize the hydrochloric acid which released from this reaction. The method was inserted by Baum. The N-benzoyl amino acids by Baum's method, in the following way [11]. 20 mmol of amino acid dissolved in 60 ml of 2N sodium hydroxide and 30 ml of water. The solution that maintained below 30°C is treated with 20 mmol of benzoyl chloride with stirring for 15-30 minutes. Then the solution acidified with 10% of hydrochloric acid until reached acidic of the solution to pH (4-5). On starting for further 30 minutes, white precipitate was formed and filtered. The benzoyl derivative is purified with methanol and dried. White crystals were obtained in good yield. The following N-benzoyl amino acids are presented in (Table 1).
| Amino acid derivative | IUPAC name | Molecular formula | Molecular weight gm/mol | Melting point (°c) | Yield % |
|---|---|---|---|---|---|
| N-Benzoyl Glycine | 2-benzamidoacetic acid | C9H9NO3 | 179 | 187-188 | 55 |
| N-Benzoyl-DL-Alanine | 2-benzamidopropanoic acid | C10H11NO3 | 193 | 164-165 | 57 |
| N-Benzoyl-D-Valine | 2-benzamido-3-methylbutanoic acid | C12H15NO3 | 221 | 126-127 | 56 |
| N-Benzoyl –DL-Isoleucine | 2-benzamido-3-methylpentanoic acid | C13H17NO3 | 235 | 115-116 | 53 |
Table 1: Structural formula, IUPAC name and physical data of N-benzoyl amino acid.
Synthesis of amides derivatives from sulfa drugs (S1,S2,S5,S8)
The new amide compounds (S1,S2,S5) prepared from corresponding N-Benzoyl amino acids and sulfa drug by means of the general procedure introduced by Curni [12]. Quantity of 1.0 mmol (0.253 gm) of sulfamethoxazole was dissolved in 50 ml of ethyl acetate to this solution 1 mmol (0.221 gm, 0.193 gm and 0.235 gm) of N-benzoyl-DValine, N-benzoyl-DL-Alanine and N-benzoyl-DL-Isoleucine respectively, were added. A solution of DCCI 1.0 mmol (0.206 gm). In 5 ml of ethyl acetate was added dropwise to the mixture solution of sulfa drugs and N-benzoyl amino acids. With governorate 15-20 minute with constant stirring at room temperature. After the addition was completed, the mixture stirred overnight. The white precipitate formed (dicyclohexyl urea) was filtered. To the filtrate solution added 10 ml of 5% aqueous solution of citric acid, two layers formed. The organic layer was extracted vigorously, the solvent evaporated and the residue was dried and then re-crystallized from ethanol (Table 2).
| Symbol of com. | Colour | Molecular formula | Molecular weight gm/mol | Melting point°c | Yield % | Elemental analysis CHNS Practical (Theoretical) |
|||
|---|---|---|---|---|---|---|---|---|---|
| S1 | White | C22H24N4O5S | 456.51 | 118-120 | 76.45 | C% | H% | N% | S% |
| 58.18 (57.88) |
5.23 (5.3) |
12.66 (12.27) |
7.35 (7.02) |
||||||
| S2 | Light brown | C20H20N4O5S | 428.46 | 188-190 | 75.5 | 56.29 (56.06) |
4.76 (4.7) |
13.14 (13.08) |
7.74 (7.48) |
| S3 | White | C18H21N3O4S | 375.44 | 196-198 | 43.6 | 57.32 (57.58) |
5.52 (5.641) |
11.07 (11.19) |
8.31 (8.54) |
| S4 | Light brown | C19H23N3O4S | 389.47 | 87-89 | 74 | 58.23 (58.59) |
5.81 (5.95) |
10.54 (10.79) |
7.97 (8.23) |
| S5 | White | C23H26N4O5S | 470.54 | 159-161 | 53.6 | 58.95 (58.71) |
5.71 (5.57) |
12.04 (11.91) |
6.93 (6.81) |
| S6 | Light brown | C16H17N3O4S | 347.39 | 148-150 | 41 | 55.63 (55.32) |
4.81 (4.93) |
12.08 (12.1) |
9.12 (9.23) |
| S7 | Orange | C15H15N3O4S | 333.36 | 97-99 | 88 | 54.65 (54.04) |
4.50 (4.54) |
12.49 (12.61) |
9.58 (9.62) |
| S8 | Yellowish-brown | C19H18N4O5S | 414.43 | 187-189 | 61.1 | 55.7 (55.06) |
4.3 (4.38) |
13.48 (13.52) |
7.78 (7.74) |
| S9 | Yellow | C20H19N5O4S | 425.46 | 80-82 | 60 | 65.72 (65.46) |
4.38 (4.50) |
16.32 (16.46) |
7.47 (7.58) |
| S10 | White | C23H25N5O4S | 467.54 | 128-130 | 44.9 | 60.12 (59.09) |
5.43 (5.39) |
14.87 (14.98) |
6.78 (6.86) |
| S11 | White | C23H24N4O4S | 452.35 | 131-133 | 54.8 | 61.35 (61.04) |
5.3 (5.35) |
12.31 (12.38) |
7.02 (7.09) |
| S12 | White | C21H21N5O4S | 439.49 | 110-112 | 85.4 | 57.61 (57.39) |
4.9 (4.82) |
15.99 (15.94) |
7.39 (7.30) |
| S13 | Yellow | C24H27N5O4S | 481.57 | 52-54 | 83.6 | 59.63 (59.86) |
5.59 (5.65) |
14.48 (14.54) |
6.21 (6.06) |
| S14 | White | C25H29N5O4S | 495.59 | 130-132 | 89 | 60.72 (60.59) |
5.87 (5.90) |
14.3 (14.13) |
6.61 (6.47) |
| S15 | Orange | C21H21N5O4S | 439.49 | 46-48 | 60.9 | 57.68 (57.39) |
4.73 (4.82) |
15.86 (15.94) |
7.4 (7.3) |
| S16 | Orange | C24H26N4O4S | 466.55 | 105-107 | 77 | 61.9 (61.78) |
5.56 (5.62) |
12.11 (12.01) |
6.91 (6.87) |
Table 2: The Symbol, Structural formula, IUPAC name, Analytical and physical data of the prepared compounds.
Synthesis of amides derivatives from sulfa drugs (S3,S4,S6,S7)
Equimolar of sulfanilamide 1 mmol (0.172 gm) and 1 mmol (0.235 gm, 0.193 gm and 0.179 gm) of N-benzoyl-DValine, N-benzoyl-DL-Isoleucine, N-benzoyl-DL-Alanine and N-benzoyl glycine respectively were dissolved in 50 ml of ethyl acetate or acetone such as in compound S7. A solution of DCCI 1 mmol (0.206 gm) in 5 ml of ethyl acetate was added gradually for 15-20 minutes with stirring to the previous solution while keeping the reaction at room temperature, the stirring continued overnight. A white precipitate formed (dicyclohexyl urea) was filtered 10 ml of 5% aqueous solution of citric acid was added to the filtrate solution, two layers formed. The organic layer was extracted power fully and the solvent evaporated. The residue which obtained was dried and re-crystallized from ethanol (Table 2).
Synthesis of amides derivatives from sulfa drugs (S9,S10,S12)
Sulfamerazine 1 mmol (0.264 gm) and 1 mmol (0.179 gm, 0.221 gm and 0.193 gm) of N-benzoyl glycine, N-benzoyl- D-Valine and N-benzoyl-DL-Alanine were mixed and dissolved in 50 ml of acetone. A solution of DCCI 1 mmol (0.206 gm) in 5 ml of ethyl acetate was added dropwise over period 15-20 minutes to the previous solution with stirring until a white precipitate formed which it was dicyclohexyl urea. The mixture left stirred overnight at room temperature. The obtained products were separated by filtration. 10 ml of 5% aqueous solution of citric acid was added to the filtrate solution. Two layers formed, the organic layer extracted vigorously, then the solvent evaporated and the residue was dried and re-crystallized from ethanol (Table 2).
Synthesis of amides derivatives from sulfa drugs (S13,S14,S15)
Equimolar of sulfamerazine 1 mmol (0.278 gm) and 1 mmol (0.221 gm, 0.235 gm and 0.179 gm) of N-benzoyl-DValine, N-benzoyl-DL-Isoleucine and N-benzoyl glycine respectively were mixed and dissolved in ethyl acetate or in acetone S15. A solution of DCCI 1 mmol (0.206 gm) in 5 ml of ethyl acetate was added dropwise over period 15-20 minutes to the previous solution with stirring until a white precipitate appears (dicyclohexyl urea). The mixture left stirred overnight at room temperature. The obtained products were separated by filtration, then 10 ml of 5% aqueous solution of citric acid was added to the filtrate solution. Two layers formed, the organic layer extracted and the residue was dried and re-crystallized from ethanol (Table 2).
Synthesis of amides derivatives from sulfa drugs (S11,S16)
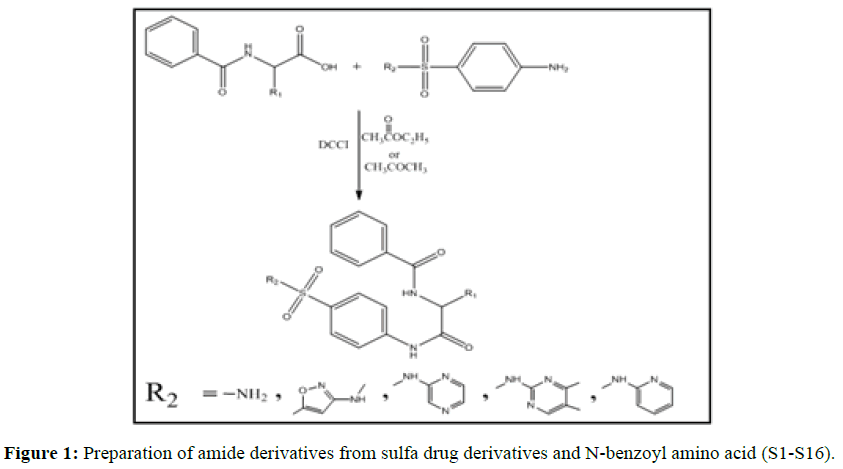
Equimolar of sulfapyridine 1 mmol (0.249 gm) and 1 mmol (0.221 gm and 0.235 gm) of N-benzoyl-D-Valine and N-benzoyl isoleucine were mixed and dissolved in 50 ml of acetone. A solution of DCCI 1 mmol (0.206 gm) in 5 ml of ethyl acetate was added dropwise over period 15-20 minutes with stirring at room temperature until whit precipitate formed (dicyclohexyl urea). The mixture lifted overnight stirring. The obtained products were separated by filtration. To the filtrate solution we added 10 ml 5% aqueous solution of citric acid was added. Two layers formed. The organic layer extracted, the residue dried and re-crystallized from ethanol (Table 2). The diversified synthetic amide derivatives compounds S1-S16 are summarized in Figure 1.
In vitro antimicrobial activity: The Antibacterial activity of the synthetic compounds S1-S16 have been carried out with two kinds of bacteria, gram positive Staphylococcus aureus (S. aureus) and gram negative Aeromonas hydrophilic (A. hydrophilia) using disc diffusion method by using Dimethylsulphoxide (DMSO) as solvent. [13] The antibiotic tetracycline 25 mg/l was having been used to calibrate and to comparison with antibacterial tools. The method includes preparing Petri plates with 10 ml of sterile nutrient agar (NA) for testing the bacterial assay. Cultivation of bacteria have been done by providing the necessary material that encourages growth at 37 °C for full day. 5 ml of nutritious broth (NB) was inoculated and incubated at 37°C for six hours until the desired bacterial growth obtained. 0.1 ml of this pure culture of each type of studied bacteria was grown in Muller Hinton Broth. Agar well was cat using glass rod and allowed to dry for minutes. Different concentration of each synthetic compounds was prepared. 0.1 ml of each solution were poured in to labeled holes of 6 mm diameter. All the plates were incubated at 37 ± 0.5°C for 24 hours. Zone of inhibition of these compounds in (mm) were registering. In addition to that, values of minimum inhibitory concentration (MIC) of these compounds were noticed. The MIC end point is the lowest concentration at which growth was observed. The tests compounds are transmitted in concentration of (25,50,100,200,250,300) mg/l. The inhibition zones created by the synthetic compounds of various micro-organisms that we used were calculated.
Results and Discussion
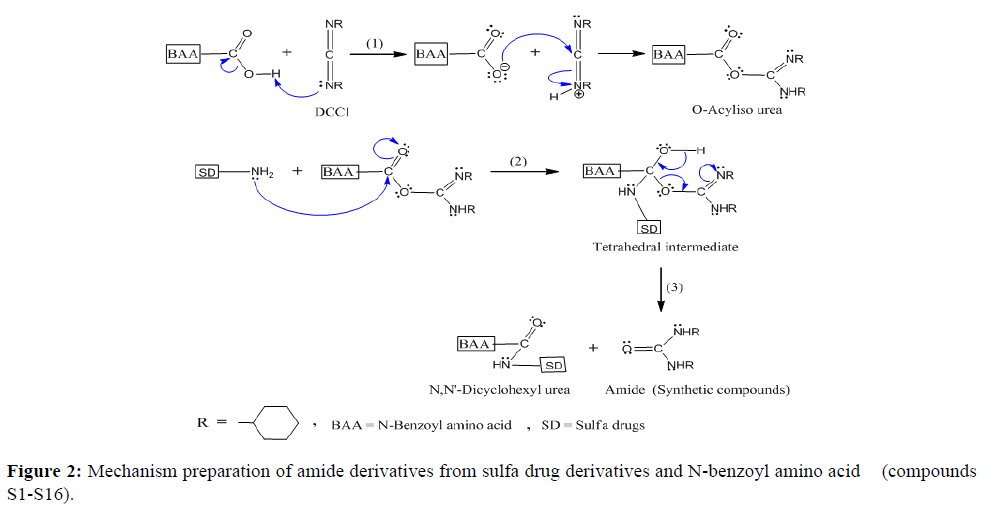
In general amides, the least reactive of the functional derivatives of carboxylic acid which can formed by nucleophilic acyl substitutions. The mechanism for connecting of an amine function (from sulfa drug derivatives) with carboxylic function (from N-benzoyl amino acid) by using DCCI can be epitomized in three steps, (Figure 2). The mechanism shows DCCI is a reagent commonly used to form amide bond which makes the free OH group of the carboxylic acid a better leaving group, therefore activating the carboxy group as a contribution to nucleophilic attack. In the first stage of the reaction, the carboxylic acid adds to DCCI (protonation). So, consists of o-acylisourea [1], the second step is the amine of sulfa drug derivatives added to the carbonyl group of o-acylisourea to give a tetrahedral intermediate [2], while the third step includes dissociated of a tetrahedral intermediate to form an amide and N,N-dicyclohexylurea. Mechanism synthesis of compounds S1-S16 are summarized in Figure 2. The outcomes of elemental analysis as it shows in (Table 2), we will find congruence with the propose structure.
Spectroscopic analysis
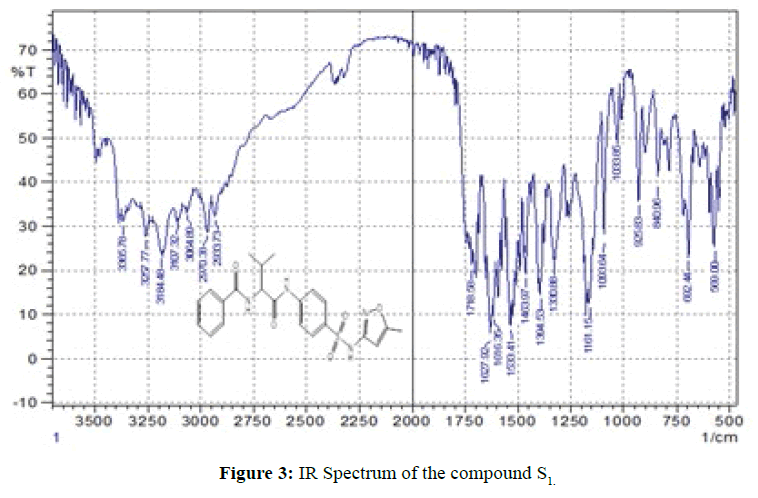
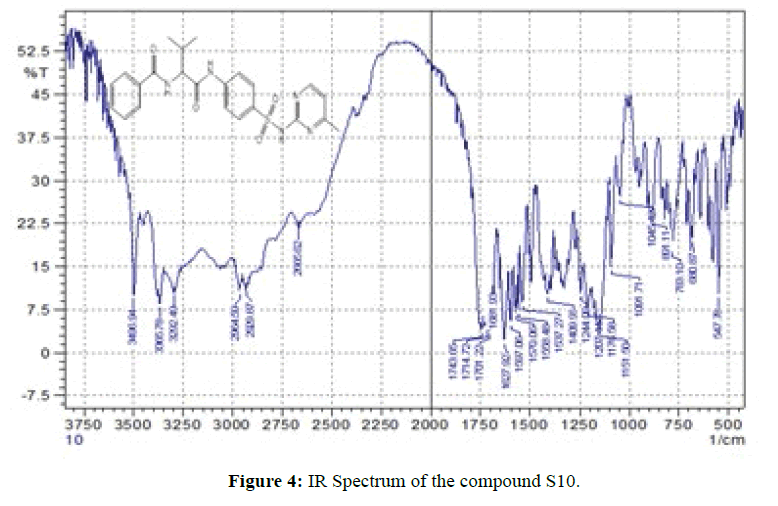
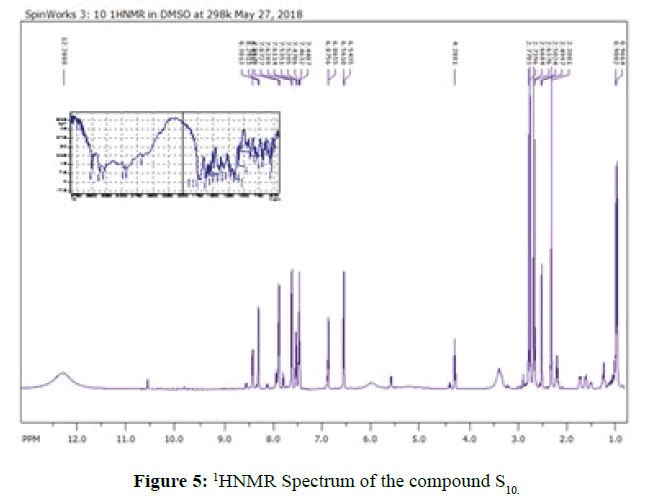
FT-IR spectra: The infrared of the synthesized compounds (S1-S16) were recorded with the range (4000-400) cm-1 and they were summarized in Table 3. The entire spectroscopic test showed expected structures. The infrared spectra show the position and the intensities of the peaks which corresponds to various groups present in each compound. The FT-IR of compounds (S1,S10) shown in Figures 3 and 4. As seen from infrared spectra of these compounds disappearance, of bands which attributed to (O-H) moiety and instead of that, the compounds display a strong-medium band at (3394- 3000) cm-1 which assigned to stretching vN-H. The formation of amide can be distinguished by appearance of strong vN-H(amide ІІ) at the range (1616-1600) cm-1 , while the (amide) І vC=Oappear as very strong bands at the range (1718-1624) cm-1. In addition, the bands present at the range (1382-1285) cm-1 and (1161-1033) cm-1 are assigned to asymmetrical and symmetric vSO2respectively [9]. Also, from IR spectra the bands appear at the range (1598-1520) cm-1 are assigned to vC=N moiety of aromatic ring in sulfa drugs [14,15]. The spectral data have been shows in Table 3.
| Com. | v(N-H) | v(C-H)Arom. Stretching | v(C-H) aliph. | v(C=O) | v(C=N) sulfa | v(S=O) Asym. | v(S=O) Sym. | v(S-N) | Sub.Arom | |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 3184 m | 3064 w | 2933 m | 1627 vs | 1533 s | 1330 m | 1161 s | 925 m | 840 m | |
| S2 | 3327 m | 3072 w | 2850 m | 1653 vs | 1593 s | 1313 w | 1163 m | 927 m | 831 w | |
| S3 | 3251 m | 3072 w | 2931 m | 1624 vs | ….. | 1313 w | 1159 m | 902 m | 827 w | |
| S4 | 3000 m | 3000 m | 2900 m | 1662 vs | ….. | 1285 w | 1045 m | 879 m | 859 w | |
| S5 | 3265 s | 3000 m | 2929 m | 1620 vs | 1537 s | 1319 s | 1033 m | 933 m | 829 w | |
| S6 | 3323 s | 3000 m | 2933 m | 1639 vs | ….. | 1307 m | 1160 w | 925 w | 825 w | |
| S7 | 3400 m | 3000 w |
2899 m | 1633 vs | ….. | 1311 m | 1045 m | 920 w | 879 w | |
| S8 | 3288 s | 3000 w | 2852 m | 1647 vs | 1521 s | 1319 m | 1168 m | 925 w | 820 w | |
| S9 | 3342 br | 3000 w | 2937 m | 1732 vs | 1598 s | 1303 m | 1161 m | 879 m | 839 w | |
| S10 | 3292 s | 3000 w | 2929 m | 1714 vs | 1597 s | 1300 m | 1151 m | 891 m | 783 w | |
| S11 | 3390 m | 3000 w | 2899 m | 1710 s | 1595 m | 1370 m | 1047 m | 879 m | 780 w | |
| S12 | 3327 s | 3000 w | 2850 m | 1627 vs | 1597 s | 1311 m | 1089 m | 893 m | 783 w | |
| S13 | 3250 m | 3000 w | 2927 m | 1635 vs | 1520 m | 1375 m | 1087 m | 920 m | 879 m | |
| S14 | 3335 m | 3061 w | 2933 m | 1683 vs | 1593 s | 1311 m | 1159 m | 975 w | 837 m | |
| S15 | 3250 m | 3000 w | 2897 m | 1653 s |
1590 m | 1313 m | 1047 m | 879 m | 780 w | |
| S16 | 3385 m | 3000 w | 2897 m | 1653 vs | 1590 m | 1382 m | 1047 w | 879 m | 785 w | |
Table 3: FT-IR data of synthetic compounds (cm-1, KBr disc) (s: strong, vs: very strong, m: medium, w: weak, br: broad).
1HNMR: The 1HNMR spectral data of all the synthesized compounds (S1-S16) were summarized in Table 4. Generally, all protons signals were found as to be in their predictable regions. The interpretation of these data gave further information and maintenance to the manner of bonding discussed in their IR spectra. All the compounds display a singlet signal at δ(12.24-12.29) ppm attributed to the proton of NHCO(amide) [16]. The multiple signals at δ(6.54-8.89) ppm can be assigned to the protons of phenyl group of these synthesized compounds. Due to 1HNMR spectrum of compounds the CH3 protons appear in different situation, as we seen in compounds S1,S2,S3,S6,S10,S12 and S13, the signal of these protons which existent in the amino acids part appeared at as a doublet signal at the range δ(0.98-1.42) ppm while the protons of CH3 of the compounds S4,S5,S14 and S16 appeared as triplet signal at the range δ(0.95-0.99) ppm and the signals of CH3 protons which is part of sulfa drugs appeared as singlet signals at the range δ(2.29-2.36)ppm as in compounds (S1,S2,S5,S8,S9,S10 and S12). Furthermore, all the signals of the groups, -CH2, CH were pointed out and indicated in the Table 4 and Figure 5. Also due to the 1HNMR spectra of the compounds S3,S4,S6 and S7 we can observed broad signal at the range δ(8.05-8.12)ppm which confirm the two protons of terminal amine group NH2 which affiliate to sulfanilamide [10,17].
| Chemical shift d (ppm) |
Structural formula | Symbol of com. |
|---|---|---|
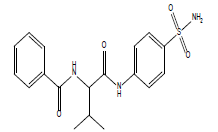
| 12.27 (br, 2H, 2NHCO) 6.54-8.4 (m, 10H, Ar) 4.28 (s, 1H, NH)sulfa 2.78 (d, 1H, CH-CO) 2.61 (m, 1H, CH) 2.31 (s, 3H, CH3)sulfa ring 1.0 (d, 6H, 2CH3) |
 |
S1 |
| 12.27 (br, 2H, 2NHCO) 6.7-8.9 (m, 10H, Ar) 4.28 (s, 1H, NH)sulfa 2.76 (q, 1H, CH-CO) 2.32 (s, 3H, CH3)sulfa ring 1.09 (d, 3H, CH3) |
 |
S2 |
| 12.29 (br, 2H, 2NHCO) 8.20 (br, 2H, NH2)sulfa 6.75-8.89 (m, 9H, Ar) 2.78 (d, 1H, CH-CO) 2.61 (m, 1H, CH) 1.05 (d, 6H, 2CH3) |
 |
S3 |
| 12.28 (br, 2H, 2NHCO) 8.15 (br, 2H, NH2)sulfa 6.72-8.50 (m, 9H, Ar) 2.77 (d, 1H, CH-CO) 2.601 (m, 1H, CH) 2.13 (m, 2H, CH2) 1.84 (d, 3H, CH3) 0.95 (t, 3H, CH3) |
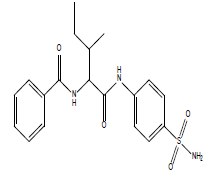 |
S4 |
| 12.28 (br, 2H, 2NHCO) 6.88-8.65 (m, 10H, Ar) 4.27 (s, 1H, NH)sulfa 2.73 (d, 1H, CH-CO) 2.29 (s, 3H, CH3)sulfa ring 2.22 (m, 2H, CH2) 2.075 (m, 1H, CH) 1.82 (d, 3H, CH3) 0.99 (t, 3H, CH3) |
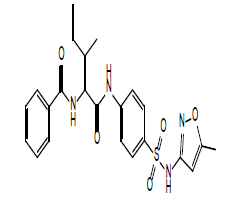 |
S5 |
| 12.29 (br, 2H, 2NHCO) 8.25 (br, 2H, NH2)sulfa 6.77-8.69 (m, 9H, Ar) 2.73 (q, 1H, CH-CO) 1.42 (d, 3H, CH3) |
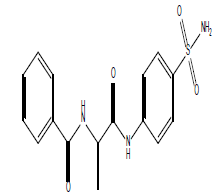 |
S6 |
| 12.27 (br, 2H, 2NHCO) 8.05 (br, 2H, NH2)sulfa 6.59-8.43 (m, 9H, Ar) 2.69 (s, 2H, CH2) |
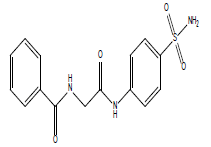 |
S7 |
| 12.24 (br, 2H, 2NHCO) 6.77-8.69 (m, 10H, Ar) 4.25 (s, 1H, NH)sulfa 2.75 (s, 2H, CH2) 2.36 (s, 3H, CH3)sulfa ring |
 |
S8 |
| Chemical shift d (ppm) |
Structural formula | Symbol of com. |
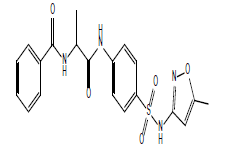
| 12.27 (br, 2H, 2NHCO) 6.65-8.71 (m, 11H, Ar) 4.25 (s, 1H, NH)sulfa 2.77 (s, 2H, CH2) 2.34 (s, 3H, CH3)sulfa ring |
 |
S9 |
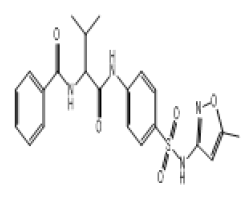
| 12.28 (br, 2H, 2NHCO) 6.54-8.30 (m, 11H, Ar) 4.28 (s, 1H, NH)sulfa 2.77 (d, 1H, CH-CO) 2.63 (m, 1H, CH) 2.30 (s, 3H, CH3)sulfa ring 0.98 (d, 6H, 2CH3) |
 |
S10 |
| 12.28 (br, 2H, 2NHCO) 6.67-8.82 (m, 13H, Ar) 4.27 (s, 1H, NH)sulfa 2.78 (d, 1H, CH-CO) 2.59 (m, 1H, CH) 1.01 (d, 6H, 2CH3) |
 |
S11 |
| 12.27 (br, 2H, 2NHCO) 6.65-8.79 (m, 11H, Ar) 4.28 (s, 1H, NH)sulfa 2.79 (q, 1H, CH-CO) 2.29 (s, 3H, CH3)sulfa ring 0.99 (d, 3H, CH3) |
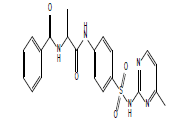 |
S12 |
| 12.25 (br, 2H, 2NHCO) 6.58-8.78 (m, 10H, Ar) 4.28 (s, 1H, NH)sulfa 2.78 (d, 1H, CH-CO) 2.56 (m, 1H, CH) 2.27 (s, 6H, 2CH3)sulfa ring 0.99 (d, 6H, 2CH3) |
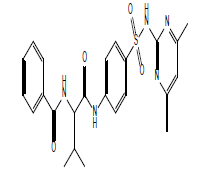 |
S13 |
| 12.27 (br, 2H, 2NHCO) 6.79-8.70 (m, 10H, Ar) 4.29 (s, 1H, NH)sulfa 2.77 (d, 1H, CH-CO) 2.29 (m, 1H, CH) 2.29 (s, 6H, 2CH3)sulfa ring 2.07 (m, 2H, CH2) 1.81 (d, 3H, CH3) 0.98 (t, 3H, CH3) |
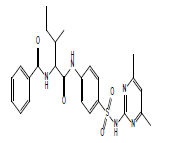 |
S14 |
| 12.29 (br, 2H, 2NHCO) 6.68-8.79 (m, 10H, Ar) 4.28 (s, 1H, NH)sulfa 2.68 (s, 2H, CH2) 2.30 (s, 6H, 2CH3)sulfa ring |
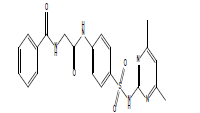 |
S15 |
| 12.27 (br, 2H, NHCO) 6.74-8.68 (m, 13H, Ar) 4.28 (s, 1H, NH)sulfa 2.78 (d, 1H, CH-CO) 2.34 (m, 1H, CH) 2.09 (m, 2H, CH2) 1.07 (d, 3H, CH3) 0.99 (t, 3H, CH3) |
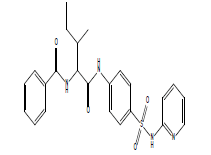 |
S16 |
Table 4: 1HNMR spectral data of synthetic compounds (S1-S16)
Antibacterial activity: In vitro antibacterial activity of the compounds (S1-S16) was done in comparison with tetracycline as standard to disclose the effectiveness of the synthesized compounds against two selected strain Gram-positive (S. aureus) and Gram-negative (A. hydrophilia). The inhibition zones were measured in mm and results are shown in Table 5. The results of antimicrobial screening indicate that these compounds show more activity against Gram-negative bacteria. A minimum inhibitory concentration (MIC) also done which can define it as the lowest concentration of the compound in a particular medium which we can located the growth of the tested strain in concentration straighten from 25-300 mg/l. Invest with data, these compounds have mutable antibacterial activity against the two species of these organism. As mentioned earlier and as a comparison to these kinds of study microorganism we can observed that these compounds have effectiveness with Gram-negative bacteria, than another kind Gram-positive especially compounds S7,S8 and S9, so the perception of antimicrobial screening denote that the synthesized compounds may be have a great effect on the cell membrane of the bacteria. According to intelligible of cell walls of bacteria, although even though that the bacterial cell wall differs from that of all other organism by the presence of peptidoglycan which is located outside of the cytoplasmic membrane and lipid bilayer membrane. In Gram-positive bacteria cell walls, the matrix substance in this wall is poly saccharides, peptidoglycan (also known as murein layer) and the major component of that wall is lipoteichoic acid, the characteristic grade for this wall thick, while the Gram-negative bacteria cell walls are thin and unlike the Gram-positive cell walls, contain a thin peptidoglycans layer . An ordinary lipid layer is very weak and may be these compounds have the ability to penetrate the cell wall and thus lead to a difference in osmotic pressure [18]. On other hand the wall membrane of the cell composed of peptidoglycans long chain of carbohydrate cross-linked by short peptides (7-12 amino acids), for this there is a possibility of some of synthesized compounds (unnatural) are incorporated into these peptides and prevents bacteria from multiplying and blocking the formation of the cross-links results in rupture of the cell [19].
| Diameter of inhibition zone (mm) Staphylococcus aureus |
Diameter of inhibition zone (mm) Aeromonas hydrophilic |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (mg/l) | Concentration (mg/l) | ||||||||||||||||
| Compounds | 25 | 50 | 100 | 200 | 250 | 300 | MIC | Compounds | 25 | 50 | 100 | 200 | 250 | 300 | MIC | ||
| S1 | 0 | 0 | 0 | 5 | 9 | 17 | 200 | S1 | 0 | 5 | 7 | 11 | 16 | 22 | 50 | ||
| S2 | 0 | 0 | 0 | 5 | 7 | 15 | 200 | S2 | 0 | 5 | 8 | 13 | 18 | 22 | 50 | ||
| S3 | 0 | 0 | 0 | 0 | 8 | 13 | 250 | S3 | 0 | 0 | 5 | 11 | 15 | 20 | 100 | ||
| S4 | 0 | 0 | 0 | 5 | 9 | 16 | 200 | S4 | 0 | 0 | 4 | 7 | 11 | 18 | 100 | ||
| S5 | 0 | 0 | 5 | 8 | 11 | 17 | 100 | S5 | 0 | 5 | 8 | 11 | 18 | 23 | 50 | ||
| S6 | O | 0 | 0 | 0 | 8 | 17 | 250 | S6 | 0 | 0 | 5 | 9 | 14 | 21 | 100 | ||
| S7 | 0 | 0 | 0 | 0 | 6 | 15 | 250 | S7 | 5 | 8 | 12 | 15 | 21 | 26 | 25 | ||
| S8 | 0 | 0 | 0 | 7 | 13 | 18 | 200 | S8 | 4 | 7 | 10 | 14 | 19 | 22 | 25 | ||
| S9 | 0 | 0 | 5 | 13 | 19 | 22 | 100 | S9 | 5 | 9 | 13 | 17 | 20 | 24 | 25 | ||
| S10 | 0 | 0 | 5 | 8 | 11 | 15 | 100 | S10 | 0 | 5 | 8 | 12 | 18 | 22 | 50 | ||
| S11 | 0 | 0 | 4 | 6 | 10 | 14 | 100 | S11 | 0 | 0 | 0 | 5 | 9 | 15 | 200 | ||
| S12 | 0 | 0 | 0 | 5 | 9 | 15 | 200 | S12 | 0 | 6 | 10 | 17 | 19 | 23 | 50 | ||
| S13 | 0 | 0 | 0 | 0 | 6 | 10 | 250 | S13 | 0 | 5 | 8 | 13 | 19 | 22 | 50 | ||
| S14 | 0 | 0 | 0 | 0 | 5 | 11 | 250 | S14 | 0 | 5 | 9 | 12 | 18 | 20 | 50 | ||
| S15 | 0 | 0 | 0 | 0 | 5 | 13 | 250 | S15 | 0 | 5 | 8 | 11 | 16 | 21 | 50 | ||
| S16 | 0 | 0 | 0 | 5 | 9 | 14 | 200 | S16 | 0 | 0 | 0 | 7 | 16 | 21 | 200 | ||
| tetracycline | 4 | 7 | 11 | 17 | 21 | 24 | 25 | tetracycline | 8 | 12 | 17 | 21 | 26 | 29 | 25 | ||
Table 5: Inhibition diameters of compounds S1-S16 against Staphylococcus aureus and Aeromonas hydrophilic.
Conclusion
Consequently, in this work we have synthesized new amide which can be considered as new sulfa drugs derivatives which in turn derived from available and well-known drugs. These synthesized compounds were characterized and confirmed by the spectroscopy methods and elemental analysis and it enhances that the reaction of sulfa drugs with benzoyl amino acid is a kind of acyl substitution reaction to form new amides compounds. This reaction updated by using DCCI as promoting reagent which accelerating the condensation reaction successfully. Also, antimicrobial activity of synthesized compounds and MIC was done in comparison with tetracycline as standard to detect the capability of the synthesized compounds. Two selected strain Staphylococcus aureus and Aeromonas hydrophilic showed good effectiveness at higher concentrations (300 mg/l), also the negative-Gram bacteria showed good sensitivity even in lowest concentration (25 mg/l).
References
- Lipmann, Hotchkiss and Dubos (1941) The occurrence of D-amino acids in gramicidin and tyrocidine. J Biol Chem 141: 163-169.
- Sari N, Arslan S, Logoglu E, Sakiyan I (2003) Antibacterial activities of some amino acid schiff bases. GUJ Sci 16: 283-288.
- Pessoa JC, Cavaco I, Correia I, Costa D, Henriques RT, et al. (2000) Preparation and characterisation of new oxovanadium (IV) Schiff base complexes derived from salicylaldehyde and simple dipeptides, Inorganica Chim Acta 305: 7-13.
- Robert J, Michal H, Lenka K, Milan N, Alexander P(2008) Characterization of Ni (II) complexes of Schiff bases of amino acids and (S)‐N‐(2‐benzoylphenyl)‐1‐benzylpyrrolidine‐2‐carboxamide using ion trap and QqTOF electrospray ionization tandem mass spectrometry. J Mass Spect 43: 1274- 1284
- Bushra KS, Raghed AG, Kahtan AA (2017) Synthesis spectral, thermal stability and bacterial activity of schiff bases derived from selective amino acid and their complexes, Adv Appl Sci Res 8: 4-12.
- Varshney A, Tandon JP (1986) Synthesis and spectral studies of tin (IV) complexes with Schiff bases derived from sulpha drugs, J Chem Sci 97: 141- 145.
- Nida Iqbal, Javed Iqbal, Muhammad Imran (2009) Base, A. Schiff. synthesis, characterizon and antibacterial studies of some metal complexes of a schiff base derived from benzaldehyde and sulfonamide. J Sci Res
- Mehta B, Mehta M (2006) Organic Chemistry , 2nd Edn, Prentice-Hall of India Private Limited, New Delhi, India.
- Bushra KAS, Heba HS,Hanaa KM (2014) Condensation of some N-phthalylaminoacid with selected sulfa drugs by using dcc as condensing agent. RJPBCS 5: 1443-1456.
- Bushra KAS (2009) DCC promoted condensation reaction of N-phthalylamino acidsWith Sulphanilamide. Mis J Acad Stud 8:18-25.
- Robert ES (1944) Benzoylation of amino acids. J Org Chem. 9: 396-400.
- Curini M, Francesco E, Federica M, Maria CM (2002) "Novel chiral Schiff base ligands from amino acid amides and salicylaldehyde, Tetrahedron Lett 43: 3821-3823.
- Jahangirian H, Haron MJ, Shah MH, Abdollahi Y, M. Rezayi et al. (2013) Well diffusion method for evaluation of antibacterial activity of copper phenyl fatty hydroxamate synthesized from canola and palm kernel oils. Dig J Nanomater Bios 8: 1263- 1270.
- Maurya RC, Chourasia J, Sharma P (2007) Synthesis, characterization and 3D molecular modeling of some ternary complexes of Cu (II), Ni (II), Co (II), Zn (II), Sm (III), Th (IV) and UO₂ (VI) with Schiff base derived from the sulfa drug sulfabenzamide and 1, 10-phenanthroline ,Ind. J. of Chem 46A: 1594- 1604.
- Neelakantan MA, Esakkiammal M, Mariappan SS, Dharmaraja J, Jeyakumar T (2010) Synthesis, characterization and biocidal activities of some schiff base metal complexes. Indian J Pharm Sci 72: 216.
- Huang Z, Wan D, Hung J (2001) Hydrolysis of Schiff Bases Promoted by UV Light, Chem Lett 708-709.
- Bushra KAS, Zainab KAK, Adil AAF (2018) Synthesis, characterization, antimicrobial activity and antioxidant of azo schiff bases containing sulphanilamide. JGPT 10: 952.
- Marye AF, James KW (2004) Organic Chemistry, 3rd Edn, Jones and Bartlett Publishers, Canada.
- Demchich P, Koch J (1996) The permeability of the wall fabric of Escherichia coli and Bacillus subtilis. Bacteriol 178: 768-773.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences