ISSN : 0976-8505
Der Chemica Sinica
Synthesis, Characterization and Antimicrobial activity of Mixed Ligand Complexes of Mn(II) and Zn(II) with Phthalic Acid or Succinic Acid and Heterocyclic Amines
Jeasmin Akter1,2*, Md. Abu Hanif1, M. Saidul Islam1, Md Masuqul Haque1, Seung Hee Lee2 and Laila Arjuman Banu1*
1Department of Chemistry, Faculty of Science, University of Rajshahi, Rajshahi, Bangladesh
2Department of BIN Convergence Technology and Department of Polymer Nano-Science and Technology, Chonbuk National University, Jeonju, Jeonbuk, Republic of Korea
Abstract
Mixed ligand complexes of Mn(II) and Zn(II) in the presence of phthalic acid or succinic acid and heterocyclic amines were synthesized and characterized. The structure of the synthesized complex was determined by elemental analysis, magnetic moment measurements, conductometric, FTIR spectral studies, and electronic spectra analysis. The complexes were showed colored, nonelectrolytic in nature. Complexes of Mn(II) were paramagnetic and Zn(II) were diamagnetic. Our results indicated that the Mn(II) complexes were octahedral, while Zn(II) complexes were of tetrahedral and octahedral structure. The synthesized complexes showed both antifungal and antibacterial activity.
Keywords
Mixed ligand complexes, Phthalic acid, Succinic acid, Heterocyclic amines, Electronic spectra, Antimicrobial activity
Introduction
For the last two decades, the synthesis of coordination compound with transition metals and their applications have become an attractive field because of the excellent properties such as catalysis, ion exchange, microelectronics, nonlinear optics, porous materials, etc. [1-7]. Due to the smaller size, and coordination numbers (4 or 6) of first row transition metal ions are easily coordinated with nitrogen and oxygen atoms and that’s why their coordination geometric structures are simple [8-14]. The mixed ligands complexes of transition metals are very important in different field of chemistry like photochemistry, analytical chemistry, magneto chemistry etc. [15].
The coordination chemistry of Mn has studied in inorganic biochemistry [16] as an interested field. The magnetic properties, diverse catalytic activity of such compounds is now being explored [17-19] for its biological importance. Recently a few reports on the antimicrobial activity of Mn(II) complexes were studied [20-24]. The complexes of Zn(II) were observed good biological and exhibit enhanced activities as compared to their parental ligands [25]. The complexes of Zn(II) showed good bacterial and fungicidal effects [26].
The synthesis of one, two or three dimensional networks [27-29] with metal complexes [30-32] succinate (C4H4O4)2 anions were used as a polydentate ligands. Phthalate acid is used as a bridging ligand to connect metal ions through the four oxygen atoms of its two carboxylate groups [33-35]. Pyridine ring containing organic compounds play an important role in many biological reactions [36].
In the present work, we describe the synthesis, characterization and antimicrobial activity of mixed ligand complexes of Mn(II) and Zn(II) with phthalic acid or succinic acid act as an primary ligands and heterocyclic amines that played as a secondary ligand. The structures of our used ligands were shown in Figure 1. The prepared complexes have also been tested in-vitro to assess their antibacterial and antifungal activities against some common reference bacteria and fungi.
Materials and Methods
Reagents and Chemicals
Manganese(II) chloride, MnCl2.4H2O (pure), zinc (II) chloride, ZnCl2.H2O (97%) were purchased from May and Baker (England). The ligands succinic acid (97%), quinoline (pure) from BDH (England) and phthalic acid (pure), pyridine (pure) obtained from Thomas and Baker (India). Solvents were purified and dried according to standard procedures.
Physical Measurements
The melting or decomposition temperatures of all the synthesized metal complexes were detected in an electro thermal melting point apparatus model SMP30. It was not possible to measure the melting points beyond 390°C. We used SHERWOOD SCIENTIFIC Magnetic Susceptibility Balance for investigating our present study. Infrared spectra (KBr) were recorded in a SHIMADZU FTIR-8400 (Japan) spectrophotometer in the range of 4000-400 cm-1. The absorbances of the complexes were noted on SHIMADZU spectrophotometer (modelUV-1800).
General Method for the Preparation of the Complexes
An ethanolic solution of metal chloride (1 mM) and deprotonated phthalic acid (1 mM) or succinic acid (1 mM) was mixed as a first ligand with constant stirring but no precipitate was observed. Ethanolic solution was used for dissolving the reactants. Then 25 mL an ethanolic potassium hydroxide solution of secondary ligand (pyridine and quinoline) in calculated ratio was mixed to the resulting mixture and heat on a magnetic regulator hot plate with constant stirring. We wait until the volume of the solution was decries to one half and allowed to cool. The precipitate was filtered, washed several times with ethanol and then dried in desiccators over anhydrous CaCl2.
Antibacterial and antifungal screening
The antibacterial and antifungal activities of the complexes were investigated against the tested bacterial species: Gram positive (Bacillus cereus and Staphylococcus aureus) and Gram negative (Escherichia coli and Shigella sonnei) and the fungal species: Candida albicans, Saccharomyces cerevisiae (Human Pathogens) and Aspergillus niger (Plant Pathogens). Ciprofloxacin and fluconazole were used as the standard antibacterial and antifungal agents. For this study diffusion method [37,38] was used. On the agar medium inoculated, the well was made with microorganisms. By using micropipette the well was filled with the test solution and the plate was incubated at 37°C for 16 h for bacteria and fungi. The test solution diffused and the growth of the inoculated microorganisms was affected during this incubated period. The zone of inhibition developed on the plate was measured. All the prepared complexes dissolved in DMSO were used for study.
Results and Discussion
Elemental analysis and conductivity measurement
The physical properties and analytical data of the Mn(II) and Zn(II)complexes are tabulated in Table 1. Elemental data of the prepared complexes were represented in Table 2. Their structures have been proposed on the basis of conductivity and magnetic moment measurements. The molar conductance’s of 1 × 10-3 M solution of the complexes in DMSO were measured at 30°C. The molar conductance values ranged from 55.3 to 99.9 ohm-1 cm2 mol-1 indicates that the compounds are non-electrolytic in nature [39,40].
| No. | Complex | Color | Decomposition temp. (± 5°C) |
Molar Conductance (ohm-1cm2mol-1) |
µeff (B.M.) |
|---|---|---|---|---|---|
| 1 | [Mn(DPA)(Py)4] | Brown | 384 | 74.8 | 1.3 |
| 2 | [Mn(DPA)(Q)4] | Black | >385 | 94.5 | 2.1 |
| 3 | [Mn(Suc)(Py)4] | Deep Brown | 270 | 89.2 | 1.5 |
| 4 | [Mn(Suc)(Q)4] | Black | >385 | 91.4 | 1.9 |
| 5 | [Zn(DPA)(Py)4] | White | 380 | 99.9 | Dia |
| 6 | [Zn(DPA)(Q)4] | Off White | 200 | 97.6 | Dia |
| 7 | [Zn(DPA)(Py)2] | White | 382 | 55.3 | Dia |
| 8 | [Zn(DPA)(Q)2] | White | 335 | 82.1 | Dia |
Where, Py=Pyridine, Q=Quinoline; DPA=Deprotonated phthalic acid, Succ=Deprotonated Succinic acid
Table 1: Physical properties and Analytical data of the complexes
| No. | Complex | Molecular Weight | %Metal | %C | %H | %N | %O | |
|---|---|---|---|---|---|---|---|---|
| 1 | [Mn(DPA)(Py)4] | Calculate | 535.45 | 8.5 | 63.27 | 4.52 | 10.47 | 11.95 |
| Found | 535.48 | 8.53 | 63.3 | 4.54 | 10.51 | 11.99 | ||
| 2 | [Mn(DPA)(Q)4] | Calculate | 735.69 | 6.19 | 72.37 | 4.38 | 7.62 | 8.7 |
| Found | 735.73 | 6.23 | 72.41 | 4.42 | 7.65 | 8.74 | ||
| 3 | [Mn(Suc)(Py)4] | Calculate | 537.77 | 8.47 | 54 | 4.5 | 10.42 | 11.9 |
| Found | 537.8 | 8.51 | 54.04 | 4.54 | 10.46 | 11.93 | ||
| 4 | [Mn(Suc)(Q)4] | Calculate | 688.01 | 6.62 | 70.35 | 4.69 | 9.3 | 8.14 |
| Found | 688.05 | 6.63 | 70.38 | 4.72 | 9.34 | 8.18 | ||
| 5 | [Zn(DPA)(Py)4] | Calculate | 544.9 | 12 | 62.18 | 4.44 | 10.28 | 11.74 |
| Found | 544.93 | 12.04 | 62.21 | 4.48 | 10.3 | 11.76 | ||
| 6 | [Zn(DPA)(Q)4] | Calculate | 746.14 | 8.76 | 71.35 | 4.32 | 7.51 | 8.58 |
| Found | 746.18 | 8.79 | 71.37 | 4.35 | 7.53 | 8.6 | ||
| 7 | [Zn(DPA)(Py)2] | Calculate | 387.70 | 16.86 | 56.18 | 3.64 | 7.23 | 16.51 |
| Found | 387.75 | 16.88 | 56.22 | 3.67 | 7.25 | 16.54 | ||
| 8 | [Zn(DPA)(Q)2] | Calculate | 487.82 | 13.40 | 64.49 | 3.72 | 5.74 | 13.12 |
| Found | 487.85 | 13.45 | 64.54 | 3.76 | 5.80 | 13.16 | ||
Table 2: Elemental analysis data of the complexes
Magnetic moment and electronic spectra
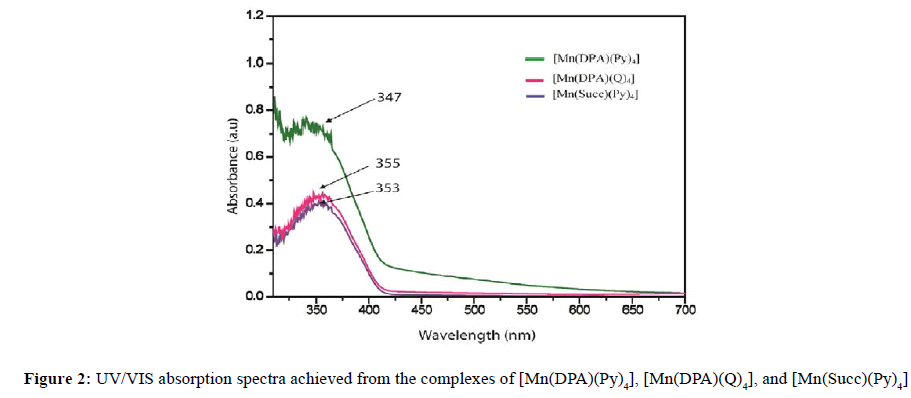
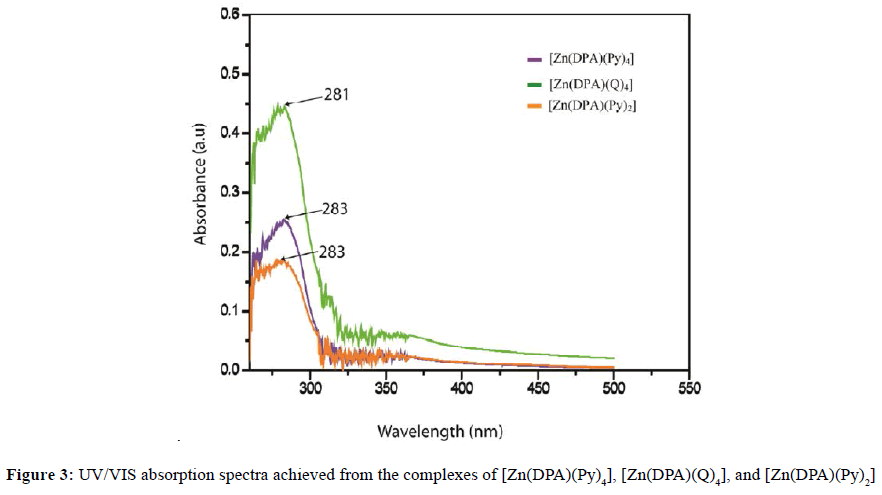
The observed values of effective moment (μeff) of the Mn(II) and Zn(II)complexes at room temperature are given in Table 1. Mn(II) complexes have μeff values (1.3-2.1 B.M.) shows the presence of one unpaired electron. So the compounds of Mn(II), under investigation are found to be paramagnetic [41]. But Zn(II) complexes have no unpaired electron. All the compounds of Zn(II), under investigation were found to be diamagnetic [42-44]. The electronic spectra of all complexes were recorded in 10-3 M DMSO at 30°C. The electronic spectra of [Mn(DPA)(Py)4], [Mn(DPA)(Q)4], [Mn(Suc)(Py)4], and [Mn(Suc)(Q)4] were shown 347, 355, 353 and 354 nm respectively, indicate octahedral geometry for the Mn(II) complex [45,46]. In Zn (II) complexes, d10 orbital are completely filled hence it does not show any d-d electronic transition but exhibit charge transfer spectra [47]. The [Zn(DPA)(Py)4], [Zn(DPA) (Q)4] and [Zn(DPA)(Py)2], [Zn(DPA)(Q)2] complexes showed bands at 283, 281 and 283, 271 nm due to the L → M charge transfer transition that correspond to octahedral [48] and tetrahedral structure [49,50]. The all data of electronic spectra of Mn(II) and Zn(II) complexes as shown in Table 3 and Figure 2 and 3.
| No. | Complex | λmax (nm) |
|---|---|---|
| 1 | [Mn(DPA)(Py)4] | 347 |
| 2 | [Mn(DPA)(Q)4] | 355 |
| 3 | [Mn(Suc)(Py)4] | 353 |
| 4 | [Mn(Suc)(Q)4] | 354 |
| 5 | [Zn(DPA)(Py)4] | 283 |
| 6 | [Zn(DPA)(Q)4] | 281 |
| 7 | [Zn(DPA)(Py)2] | 283 |
| 8 | [Zn(DPA)(Q)2] | 271 |
Table 3: Data for the determination of UV-visible spectral bands Antibacterial and antifungal screening
FTIR studies
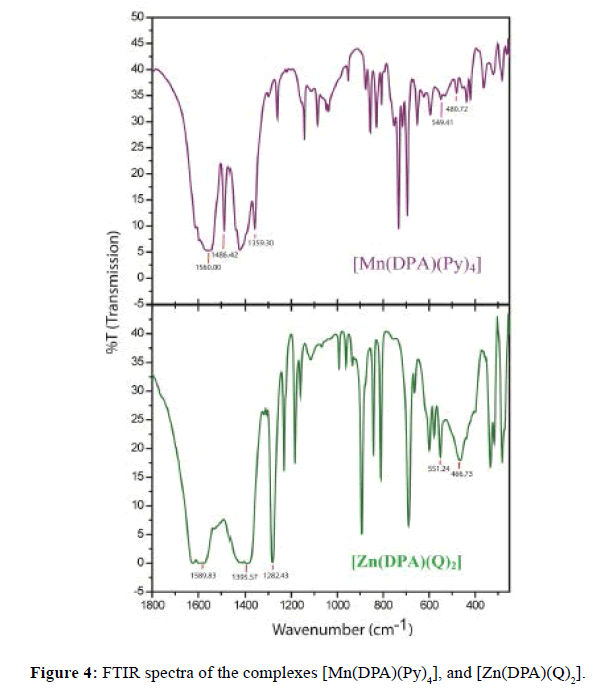
FTIR spectra of the Mn(II) and Zn(II) complexes were shown in Table 4 and Figure 4. The complexes display bands in the regions (1520-1600) and (1280-1390) cm-1 due to υ(C=O) and υ(C-O) respectively, significantly lower than that of free ligand indicating the coordination of metal ion through its carboxylate anion. The band observed in the (1395- 1605) cm-1 region due to υ(C=N) vibrations. The in-plane and out-of-plane ring deformation modes of heterocyclic amines observed at 680 and 620 cm-1 respectively undergo a positive shift in mixed ligand complexes confirming their coordination through nitrogen. The presence of metal nitrogen bonding in the complexes is evident from the appearance at 456-690 cm-1 region in the spectra of the complexes of υ(M-N) modes [51] and υ(M-O) appearance at (543-690) cm-1.
| No. | Complex | υ(C=O) | υ(C-O) | υ(C=N) | υ(M-O) | υ(M-N) |
|---|---|---|---|---|---|---|
| 1 | [Mn(DPA)(Py)4] | 1560.00 | 1359.30 | 1486.42 | 549.41 | 480.72 |
| 2 | [Mn(DPA)(Q)4] | 1530.54 | 1298.56 | 1498.67 | 654.12 | 499.32 |
| 3 | [Mn(Suc)(Py)4] | 1598.98 | 1380.45 | 1545.23 | 543.98 | 460.32 |
| 4 | [Mn(Suc)(Q)4] | 1576.87 | 1298.65 | 1467.78 | 687.34 | 466.12 |
| 5 | [Zn(DPA)(Py)4] | 1575.23 | 1308.87 | 1604.4 | 632.23 | 498.23 |
| 6 | [Zn(DPA)(Q)4] | 1588.34 | 1390.65 | 1585.8 | 609.12 | 456.12 |
| 7 | [Zn(DPA)(Py)2] | 1521.12 | 1376.45 | 1578.2 | 543.43 | 502.76 |
| 8 | [Zn(DPA)(Q)2] | 1589.83 | 1282.43 | 1395.57 | 551.24 | 466.73 |
Table 4: Data for the determination of IR Spectroscopy in cm-1
Metal complexes play an important role in regulating biological activities. The antimicrobial activity is depending on the cell membrane of the microorganisms and nature of metal ions [52]. The uses of metals for preventing or reducing growth of bacterial and fungal in medical treatment have been reported [53]. The antibacterial and antifungal potentiality of all the tested compounds against the four chosen bacteria and three fungi were shown in Tables 5 and 6. The zone of inhibition (mm) around the discs was measured. Results are expressed as zone of inhibition. The results of the antibacterial and antifungal screening indicated that all the compounds exhibit broad spectra against the reference bacteria and fungi.
| Complex | Zone of inhibition, diameter in mm | |||
|---|---|---|---|---|
| Bacillus cereus | Staphyllococeus aureus |
Escherichia coli | Shigella sonnei | |
| [Mn(DPA)(Q)4] | 12 | 6 | 10 | 10 |
| [Mn(DPA)(Py)4] | 7 | 6 | 10 | 8 |
| [Zn(DPA)(Py)4] | 18 | 12 | 12 | 8 |
| [Zn(DPA)(Q)2] | 6 | 6 | 11 | 6 |
| Ciprofloxacin | 30 | 40 | 30 | 40 |
Table 5: Antibacterial activity of the synthesized complexes
| Complex | Zone of inhibition, diameter in mm | ||
|---|---|---|---|
| Candida albicans | Aspergllus niger | Saccharromycescerevaceae | |
| [Mn(DPA)(Q)4] | 8 | 16 | 14 |
| [Mn(DPA)(Py)4] | 12 | 12 | 12 |
| [Zn(DPA)(Q)2] | 20 | 16 | 12 |
| [Zn(DPA)(Py)4] | 12 | 15 | 18 |
| Fluconazole | 12 | 12 | 12 |
Table 6: Antifungal activity of the synthesized complexes
Conclusion
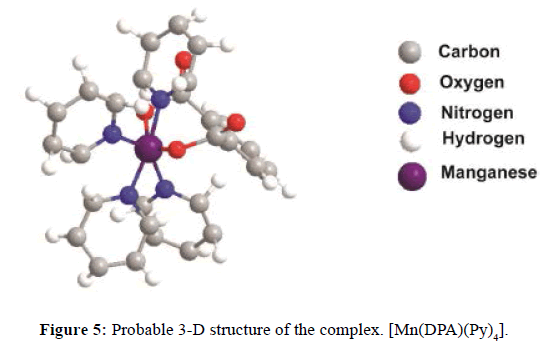
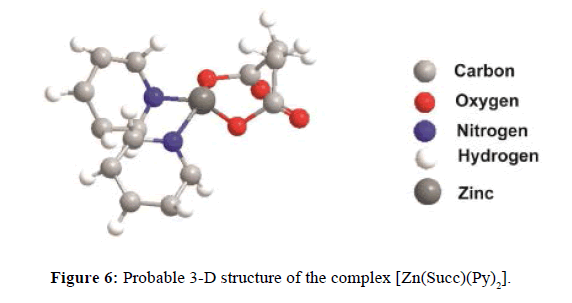
On the basis of the above analysis, we have proposed octahedral geometry for Mn(II) complex but tetrahedral, and octahedral geometry both for Zn(II) complexes. The proposed structures of [Mn(DPA)(Py)4], and [Zn(Succ)(Py)2] have presented in Figure 5 and Figure 6. All the prepared complexes observed good antimicrobial activity. The tested mixed-ligand complexes showed higher activities against fungi compared to bacteria.
Acknowledgements
The authors are thankful to National Science and Technology Ministry (NST), Bangladesh for their financial support. The authors are also grateful to the Department of Chemistry, Faculty of Science, University of Rajshahi, Rajshahi, Bangladesh.
References
- Matsuda R, Kitaura R, Kitagawa S, Kubota Y, Belosludov RV, et al. (2005) Highly controlled acetylene accommodation in a metal–organic microporous material. Nature 436: 238-241.
- Chui SSY, Lo SMF, Charmant JPH, Orpen AG (1999) A chemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3]n. Science 283: 1148-1150.
- Moulton B, Zaworotko MJ (2001) From molecules to crystal engineering: supramolecular isomerism and polymorphism in network solids. Chem Rev 101: 1629-1658.
- Kitagawa S, Kitaura R, Noro S (2004) Functional porous coordination polymers. Angew Chem Int 43: 2334-2375
- Blake AJ, Champness NR, Hubberstey P, Li WS, Withersby MA, et al. (1999) Inorganic crystal engineering using self-assembly of tailored building-blocks. Coord Chem Rev 183: 117-138.
- Chen BL, Wang LB, Zapata F, Qian GD, Lobkovsky EB (2008) A luminescent microporous metal− organic framework for the recognition and sensing of anions. J Am Chem Soc 130: 6718-6719.
- Horcajada P, Serre C, Maurin G, Ramsahya NA, Balas F, et al. (2008) Flexible porous metal-organic frameworks for a controlled drug delivery. J Am Chem Soc 130: 6774-6780.
- Paz FAA, Klinowski J (2004) Two- and three-dimensional cadmium-organic frameworks with trimesic acid and 4,4‘-trimethylenedipyridine. Inorg Chem 43: 3882-3893.
- Withersby MA, Hlake AJ, Champness NR, Cooke PA, Hubberstey P, et al. (1999) Schröder M, Parallel interpenetration in novel herringbone sheets formed by Co(II) and Cd(II) complexes with trans-4,4′-azobis(pyridine). New J Chem 23: 573-575.
- Coronado E, Galán M JR, Gómez GCJ, Martínez FE, Smaalen SV (2004) Incommensurate nature of the multilayered molecular ferromagnetic metals based on bis(ethylenedithio)tetrathiafulvalene and bimetallic oxalate complexes. Inorg Chem 43: 4808.
- Maji TK, Mostafa G, Matsuda R, Kitagawa S (2005) Guest-induced asymmetry in a metal-organic porous solid with reversible single-crystal-to-single-crystal structural transformation. J Am Chem Soc 127: 17152-17153.
- Lu YL, Wu JY, Chan MC, Huang SM, Lin CS, et al. (2006) Influence of water content on the self-assembly of metal− organic frameworks based on pyridine-3, 5-dicarboxylate. Inorg Chem 45: 2430-2437.
- Wang ZM, Zhang B, Kurmoo M, Green MA, Fujiwara H, et al. (2005) Synthesis and characterization of a porous magnetic diamond framework, Co3(HCOO)6, and its N2 sorption characteristic. Inorg Chem 44: 1230-1237.
- Rujiwatra A, Kepert CJ, Rosseinsky MJ (1999) The organo-pillared porous magnetic framework CO4(SO4)(OH)6(H2NC2H4NH2)0.5•3H2O. Chem Commun 22: 2307-2308.
- International tables for X-ray crystallography (1974) vol. IV, Kynoch Press, Birmingham.
- Pecoraro VL, Hsieh WY, Sigel A, Sigel H, Marcel Dekker (2000) Metal ions in biological systems: manganese and its role in biological processes. New York, 37: 431.
- McGarrigle EM, Gilheany DG (2005) Chromium and manganese salen promoted epoxidation of alkenes. Chem Rev 105: 1563-1602.
- Kahn O (1993) Molecular magnetism. VCH, Weinheim, Germany.
- Mandal S, Rout AK, Fleck M, Pilet G, Ribas J, et al. (2010) Synthesis, crystal structure and magnetic characterization of a series of four phenoxo-bridged binuclear manganese(III) Schiff base complexes. Inorg Chim Acta 363: 2250-2258.
- Dorkov P, Pantcheva IN, Sheldrick WS, Mayer-Figge H, Petrova R, et al. (2008) Synthesis, structure and antimicrobial activity of manganese (II) and cobalt (II) complexes of the polyether ionophore antibiotic Sodium Monensin A. J Inorg Biochem 102: 26-32.
- Geraghty M, Cronin JF, Devereux M, McCann M (2000) Synthesis and antimicrobial activity of copper (II) and manganese (II) α, ω-dicarboxylate complexes. BioMetals 13: 1-8.
- Patil M, Hunoor R, Gudasi K (2010) Transition metal complexes of a new hexadentate macroacyclic N2O4-donor Schiff base: inhibitory activity against bacteria and fungi. Eur J Med Chem 45: 2981-2986.
- Mohamed GG, Omar MM, Ibrahim AA (2010) Preparation, characterization and biological activity of novel metal-NNNN donor Schiff base complexes. Spectrochim Acta A Mol Biomol Spectrosc 75: 678-685.
- Nishat N, Rahis-Ud-Din, Dhyani S (2009) Synthesis, characterization and antimicrobial activity of a new macrocycle and its transition metal complexes. J Coord Chem 62: 996-1004.
- Singh AK, Pandey OP, Sengupta SK (2012) Synthesis, spectral characterization and biological activity of zinc (II) complexes with 3-substituted phenyl-4-amino-5-hydrazino-1, 2, 4-triazole Schiff bases. Spectrochim Acta Part A 85: 1-6.
- Hanif MA, Zahid AASM, Akter J, Islam MS, Haque MM, et al. (2016) Synthesis, characterization and antimicrobial activity of Ni(II) and Zn(II) complexes with amino acids and heterocyclic amine. Der Chem Sin 7: 75.
- Livage C, Egger C, Nogues M, Ferey G (1998) Hybrid open frameworks (MIL-n). Part 5 Synthesis and crystal structure of MIL-9: a new three-dimensional ferrimagnetic cobalt(II) carboxylate with a two-dimensional array of edge-sharing Co octahedra with 12-membered rings. J Mater Chem 8: 2743-2747.
- Livage C, Egger C, Ferey G (1999) Hybrid Open Networks (MIL 16): Synthesis, Crystal Structure, and Ferrimagnetism of Co4(OH)2(H2O)2(C4H4O4)3•2H2O, A new layered cobalt(II) carboxylate with 14-membered ring channels. Chem Mater 11: 1546-1550.
- Livage C, Egger C, Ferey G (2001) Hydrothermal versus nonhydrothermal synthesis for the preparation of organic-inorganic solids: the example of cobalt(II) succinate. Chem Mater 13: 410-414.
- Vaidhyanathan R, Natarajan S, Rao CNR (2001) Three-dimensional yttrium oxalates possessing large channels. J Chem Mater 13: 185-191.
- Prasad PA, Neeraj S, Natarajan S, Rao CNR (2000) Synthesis and structure of the first open-framework cadmium oxalate possessing channels. Chem Commun 14: 1251-1252.
- Zhang J, Li ZJ, Wen YH, Kang Y, Cheng JK, et al. (2004) Synthesis and crystal structures of two metal succinates modified by bipyridines. Z Anorg Allg Chem 630: 2731-2735.
- Bakalbassis EG, Bozopoulos AP, Mrozinski J, Rentzeperis PJ, Tsipis CA (1988) Crystal structure, magnetic properties, and orbital interactions of the [(mu-terephthalato)(ethylenediamine) diaquocopper (II)] zigzag chain. Inorg Chem 27: 529.
- Balkalbassis EG (1994) A novel 2D polymeric structure of Cu–Na complex, in which phthalate gives seven coordination sites. Inorg Chim Acta 218: 167.
- Jiang ZH, Ma SL, Liao DZ, Yan SP, Wang GL (1993) Synthesis and crystal structure of a novel tetragonal Mn(II) complex with ferromagnetic coupling. Sic China (Ser. B) 23: 1009.
- Abd EMG (2003) Thermodynamic and structural studies of complexes of manganese (II), cobalt (II), nickel (II) and copper (II) with aminofuropyridine carboxamide. J Serb Chem Soc 68: 463-470.
- Bauer AW, Kirby WM, Sherris JC, Turck M (1966) Antibiotic susceptibility testing by a standardized single disk method. Am J ClinPathol 45: 493-496.
- Patil SS, Thakur GA, Patil VR (2009) Synthesis, spectral and biological studies on some mixed ligand Ni (II) complexes. Acta Pol Pharm Drug Res 66: 271-277.
- Natarajan R, Antonysamy K, Chinnathangavel T (2003) Redox and antimicrobial studies of transition metal (II) tetradentate Schiff base complexes. Transit Metal Chem 28: 29-36.
- Mohamed GG, Gamel NEAEL (2004) Synthesis, investigation and spectroscopic characterization of piroxicam ternary complexes of Fe(II), Fe(III), Co(II), Ni(II), Cu(II) and Zn(II) with glycine and dl-phenylalanine. Spectrochim Acta Part A 60: 3141-3154.
- Pragnesh PK, Patel MN (2004) Synthesis, Structural Characterization, and Antibacterial Studies of Some Mixedâ€ÂÂLigand First Row dâ€ÂÂTransition Metal Complexes. Syn React Inorg Met-Org Chem 34: 1277-1289.
- Ali MA, Haroon CM, Nazimuddin M, Majumder SMMH, Tarafder MTH (1992) Synthesis, characterization and biological activities of some new nickel (II), copper (II), zinc (II) and cadmium (II) complexes of quadridentate SNNS ligands. Trans Met Chem 17: 133-138.
- Maurya RC, Patel P, Rajput S (2003) Synthesis and characterization of mixedâ€ÂÂligand complexes of Cu(II), Ni(II), Co(II), Zn(II), Sm(III), and U(VI)O2, with a Schiff base derived from the sulfa drug sulfamerazine and 2,2′â€ÂÂbipyridine. MetOrg Chem 33: 801-816.
- Serbest K, Kayi H, Er M, Sancak K (2008) Degirmencioglu I, Ni(II), Cu(II), and Zn(II) complexes of tetradentate schiff base containing two thiadiazoles units: Structural, spectroscopic, magnetic properties, and molecular modeling studies. Heteroatom Chem 19: 700.
- Shelke VA, Jadhav SM, Shankarwar SG, Munde AS, Chondhekar TK (2011) Synthesis, characterization, antibacterial and antifungal studies of some transition and rare earth metal complexes of N-benzylidene-2-hydroxybenzohydrazide. Bull Chem Soc Ethiop 25: 381-382.
- Cheikh HK, Daniel T, Farba BT, Ibrahima ET, Aliou HB, et al. (2016) Synthesis and characterization of novel M(II) (M = Mn(II), Ni(II), Cu(II) or Zn(II)) complexes with tridentate N2,O-donor ligand (E)-2-amino-N’-[1-(pyridin-2 yl)ethylidene]benzohydrazide. Bull Chem Soc Ethiop 30: 101-110.
- Nair MS, Arish D, Joseyphus RS (2012) Synthesis, characterization, antifungal, antibacterial and DNA cleavage studies of some heterocyclic Schiff base metal complexes. J Saudi Chem Soc 16: 83-88.
- Kiran S, Manjeet SB, Parikshit T (2007) Synthesis and characterization of cobalt(II), nickel(II), copper(II) and zinc(II) complexes with Schiff base derived from 4-amino-3- mercapto-6-methyl-5-oxo-1,2,4-triazine. Eur J Med Chem 42: 394-402.
- Sharma PK, Sen AK, Singh K, Dubey SN (1997) Divalent cobalt, copper and zinc complexes of n salicylideneamino acids. J Ind Chem Soc 74: 446-447.
- Temel H, Ilhan S, Sekerci M, Ziyadanogullari R (2002) The synthesis and spectral characterization of new Cu (II), Ni (II), Co (III), and Zn (II) complexes with Schiff base. Spectroscopy Lett 35: 219-228.
- Nakamoto K (1970) Infrared spectra of inorganic and coordination compounds. Wiley Interscience, New York.
- Joseyphus RS, Nair MS (2010) Synthesis, characterization and biological studies of some Co(II), Ni(II) and Cu(II) complexes derived from indole-3-carboxaldehyde and glycylglycine as Schiff base ligand. Arabian J Chem 3: 195-204.
- Kateřina M, Petr P, Zuzana R, Ondřej K (2011) Antibacterial and antifungal activities of silver, copper and zinc montmorillonites. Appl Clay Sci 53: 642-645.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences