ISSN : 0976-8505
Der Chemica Sinica
Synthesis and Structural Characterization of Meso-tetrakis(3,4-dimethoxyphenyl)Porphyrin
Department of Applied Chemistry, Delhi Technological University, Delhi-42, India.
Abstract
Sterically hindered and electron releasing group attached on meso phenyl containing porphyrin are of great interest in the scientific community. This report emphasize on the synthetic as well as structural characterization of mesotetrakis( 3,4-dimethoxyphenyl)porphyrin.
Keywords:
porphyrin, crystal, methoxy, synthesis
Introduction
Porphyrins and their analogues materials serve different applications. Metalloporphyrins assemblies has been used as building block for tailored materials properties during the past decades the [1]. These fascinating molecules have broad applications as field responsive materials, particularly for optoelectronic applications [2-4]. The facile substitution of the periphery of various porphyrins has generated a series of unusual liquid crystalline materials [5-8]. The porphyrins ligand serves as a platform on which one can erect desirable molecular and materials properties, including very large dipole moments [9], polarizabilities [10], and hyperpolarizibilities [11-13]. The nonlinear optical properties of these materials can be explored in optical communications, data storage, and electro-optical signal processing [14-18]. The porphyrin π-radical makes these systems especially interesting for photoionization processes [19-25], closely related to the so-called ion pair reaction centre of photosynthesis and the photo-generation of electron transfer [26]. Porphyrins can interact with chemical species (chemo-responsive) also in contrast to their interaction with applied electric [27], magnetic or electromagnetic fields. The materials based on chemo-responsive are less advanced e.g. porphyrins solids, being highly porous, are involved in the current development of molecular based molecular sieves or shape- selective solid catalysts and in sensor applications [28-31]. In this paper, we have reported the single crystal structure of sterically hindered porphyrin for future applications.
Experimental
Materials and methods
The synthesis of 3, 4-dimethoxytetraphenylporphyrin was carried using reported method [22] and details are as 1.0 g (6.0 mmol) of 3, 4-dimethoxybenzaldehyde was dissolved in 50 ml of argon-purged dimethylformamide. 0.5 mL conc. HCl was added into it followed by drop wise addition of 0.42 mL (6.0 mmol) of freshly distilled pyrrole. The mixture was stirred under argon for a 1 hour. The final reaction mixture was refluxed for 8 hour in air. The solvent was evaporated on water bath and washed with hot water. The crude product was extracted by flash chromatography over silica gel using 10% ethyl acetate-chloroform mixture. The desired porphyrin was finally purified by column chromatography over silica gel using 7% ethyl acetate-chloroform mixture. The yield of the purple crystal compound was calculated to 25%.
Analytical data
1H (CDCl3, 400MHz); δ-2.81 (br s, -NH, pyrrole), 3.93 (s, 12H, m-OCH3), 4.11 (s, 12H, p-OCH3), 7.17 (d, 4H, phenyl), 7.67 (d, 4H, phenyl), 7.70 (s, 4H, phenyl), 8.83 (s, 8H, pyrrole); 13C (CDCl3, 400MHz); 56.2 (m-OCH3), 56.3 (p-OCH3), 127.46 (meso-carbon), 134.85 (β-C, pyrrole), 147.1 (α-C, pyrrole), 109.51, 118.3, 119.9, 148.95; λmax (CHCl3: logε); 425 (5.63), 464 (4.17), 519 (4.28), 559 (4.04), 593 (3.78), 649 (3.14); FAB mass (m/z): 856.
Results and Discussion
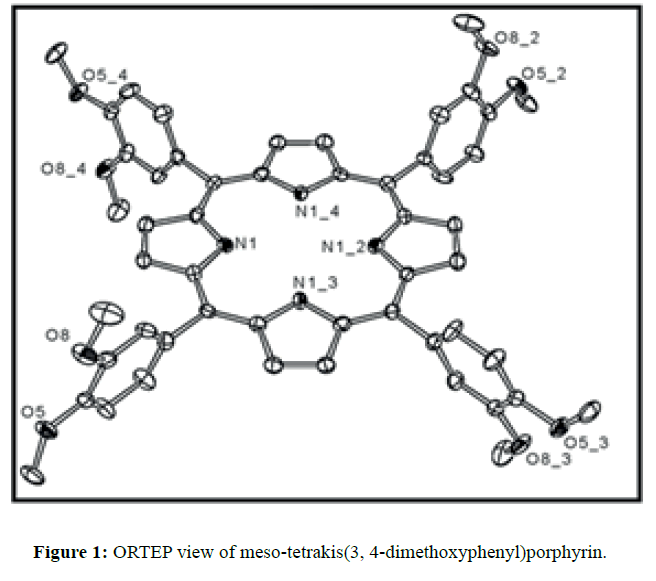
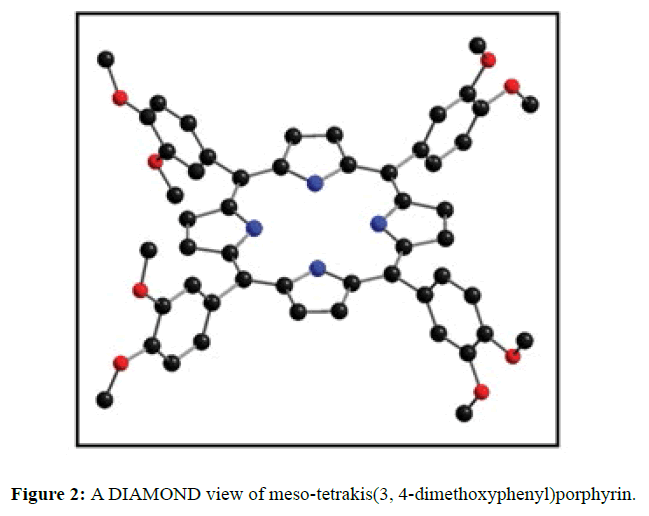
The diffraction quality single crystals of meso-tetrakis(3,4-dimethoxyphenyl)porphyrin were grown by direct solvent diffusion of n-hexane in a saturated solution of porphyrin in chloroform in a long cylindrical tube standing on several days at room temperature. A dark purple rectangular block shaped single crystal of meso-tetrakis(3,4-dimethoxyphenyl) porphyrin having approximate dimensions of crystal size 0.20 × 0.10 × 0.07 mm was mounted for data collection. A total no. of 17352 reflections (2.20<Q<28.33ο) were collected of which 6395 no. of unique reflections (R (int) 0.0612) were used. This crystal was solved under the space group of I-4 in tetragonal system. Final R=0.0634 for 6395 no of unique reflections with I>2σ Max/min residual density 0.470 and -0.268 e Å-3. The asymmetric unit (Z=4) contains two one fourth of two structurally different porphyrin molecules which are separated by each other and one disordered chloroform solvent molecule. The two Cl atoms of chloroform are disordered over four positions (Cl1A, Cl1B, Cl2A and Cl2B) and were refined using free part variable, due the disorderness of the solvent we are unable to put the hydrogen atom in chloroform. The ORTEP and DIAMOND views of free base porphyrin are shown in Figures 1 and 2 respectively where hydrogen atoms are omitted due to clarity. The anisotropic parameters for all nonhydrogen atoms are listed below in Table 1. The selected bond lengths and bond angles are summarized in Table 2. A crystallographic data is tabulated in the Table 3.
| X | Y | Z | U(eq) | |
|---|---|---|---|---|
| O(2) | 892(1) | 2589(1) | 6794(1) | 23(1) |
| O(3) | -85(1) | 1962(1) | 7696(1) | 25(1) |
| N(1) | 5655(2) | 3966(2) | 4998(2) | 18(1) |
| O(5) | 3712(2) | -286(1) | 4971(1) | 27(1) |
| O(8) | 3735(2) | 493(2) | 3763(1) | 30(1) |
| C(5) | 4546(2) | 2991(2) | 5002(2) | 17(1) |
| C(17) | 3076(2) | 757(2) | 7551(2) | 15(1) |
| C(21) | 1208(2) | 1986(2) | 7204(2) | 18(1) |
| N(10) | 3872(2) | -491(2) | 7504(1) | 16(1) |
| C(4) | 5353(2) | 3206(2) | 4978(2) | 19(1) |
| C(11) | 4170(2) | 1719(2) | 4360(2) | 23(1) |
| C(19) | 2240(2) | 1079(2) | 7613(2) | 17(1) |
| C(22) | 671(2) | 1648(2) | 7703(2) | 19(1) |
| C(3) | 6004(2) | 2648(2) | 4935(2) | 21(1) |
| C(23) | 932(2) | 1051(2) | 8160(2) | 20(1) |
| C(6) | 4348(2) | 2116(2) | 5005(2) | 20(1) |
| C(24) | 1714(2) | 770(2) | 8109(2) | 21(1) |
| C(10) | 3966(2) | 925(2) | 4363(2) | 23(1) |
| C(14) | 2854(2) | -1356(2) | 7261(2) | 20(1) |
| C(20) | 1980(2) | 1693(2) | 7152(2) | 17(1) |
| C(1) | 6473(2) | 3909(2) | 4976(2) | 17(1) |
| C(16) | 3164(2) | -71(2) | 7460(2) | 15(1) |
| C(25) | 1421(2) | 2965(2) | 6290(2) | 31(1) |
| C(15) | 2533(2) | -617(2) | 7297(2) | 17(1) |
| C(12) | 3554(3) | -680(2) | 5635(2) | 33(1) |
| C(18) | 3719(2) | 1304(2) | 7599(2) | 14(1) |
| C(9) | 3953(2) | 498(2) | 5018(2) | 21(1) |
| C(8) | 4148(2) | 879(2) | 5648(2) | 27(1) |
| C(2) | 6694(2) | 3074(2) | 4941(2) | 23(1) |
| C(7) | 4343(2) | 1691(2) | 5646(2) | 27(1) |
| C(26) | -692(2) | 1513(3) | 8061(2) | 29(1) |
| C(13) | 3705(3) | 902(3) | 3095(2) | 43(1) |
| Cl(3A) | 8520(7) | 1834(6) | 5964(10) | 91(3) |
| Cl(3B) | 8429(4) | 1895(3) | 5811(6) | 37(3) |
| Cl(2A) | 9254(2) | 1960(2) | 4593(1) | 57(1) |
| Cl(2B) | 9849(3) | 1748(2) | 4959(3) | 98(2) |
| Cl(1A) | 9640(2) | 824(2) | 6241(2) | 68(1) |
| Cl(1B) | 10095(2) | 1127(2) | 5709(2) | 69(2) |
| C(27A) | 9445(3) | 1829(3) | 5668(4) | 85(2) |
Table 1: Atomic coordinates (X 104) and equivalent isotropic displacements parameters (Å2 x 103) for meso-tetrakis(3, 4-dimethoxyphenyl)porphyrin. U(eq) is defined as one third of the trace of the orthogonolized Uij tensor
| Bond distance | |||
| O(2)-C(21) | 1.367(4) | N(10)-C(18)#2 | 1.366(4) |
| O(2)-C(25) | 1.432(4) | N(10)-C(16) | 1.378(4) |
| O(3)-C(22) | 1.369(4) | N(1)-C(4) | 1.367(4) |
| O(3)-C(26) | 1.432(4) | N(1)-C(1) | 1.371(4) |
| O(5)-C(9) | 1.372(4) | O(8)-C(10) | 1.380(4) |
| O(5)-C(12) | 1.419(4) | O(8)-C(13) | 1.415(5) |
| C(17)-C(18) | 1.413(5) | C(17)-C(16) | 1.402(4) |
| C(18)-N(10)#4 | 1.366(4) | Cl(3A)-C(27A) | 1.640(9 |
| Cl(3B)-C(27A) | 1.722(8) | Cl(2A)-C(27A) | 2.026(8) |
| Cl(2B)-C(27A) | 1.482(7) | Cl(2B)-Cl(1B) | 1.781(7) |
| Cl(1A)-C(27A) | 2.014(7) | Cl(1B)-C(27A) | 1.599(6) |
| C(27A)-H(27A) | 0.98 | C(18)-C(14)#4 | 1.438(4) |
| Bond angles | |||
| C(21)-O(2)-C(25) | 116.5(3) | C(22)-O(3)-C(26) | 116.7(3) |
| C(4)-N(1)-C(1) | 107.6(3) | C(9)-O(5)-C(12) | 116.2(3) |
| C(10)-O(8)-C(13) | 117.4(3) | C(18)#2-N(10)-C(16) | 107.4(2) |
| N(1)-C(4)-C(5) | 126.5(3) | N(1)-C(4)-C(3) | 109.0(3) |
| N(1)-C(1)-C(5)#3 | 126.0(3) | N(1)-C(1)-C(2) | 108.8(3) |
| N(10)#4-C(18)-C(17) | 126.0(3) | N(10)#4-C(18)-C(14)#4 | 108.7(3) |
| C(8)-C(9)-O(5) | 124.6(3) | O(5)-C(9)-C(10) | 115.8(3) |
Table 2: Selected bond distance (Å) and bond angles (deg) for meso-tetrakis(3, 4-dimethoxyphenyl)porphyrin.
| Parameters | Compound |
|---|---|
| Empirical formula | C54 H46 Cl6 N4 O8 |
| Formula weight | 1091.65 |
| Temperature | 100(2) K |
| Wavelength | 0.71069 Å |
| Crystal system | Tetragonal |
| Space group | I-4 |
| a | 16.713(5) Å |
| b | 16.713(5) Å |
| c | 18.507(5) Å |
| α | 90.000(5)ο |
| β | 90.000(5)ο |
| γ | 90.000(5)ο |
| Volume | 5169(3) Å3 |
| Z | 4 |
| Density (calculated) | 1.403 Mg/m3 |
| Absorption coefficient | 0.391 mm-1 |
| F (000) | 2256 |
| Refinement method | Full-matrix least-squares on F2 |
| Goodness-of-fit on F2 | 1.042 |
| Final R indices [I>2σ(I)] | R1 = 0.0634, wR2 = 0.1240 |
| R indices (all data0 | R1 = 0.0932, wR2 = 0.1365 |
Table 3: Summary of X-ray crystallographic data for complexes meso-tetrakis(3, 4-dimethoxyphenyl)porphyrin.
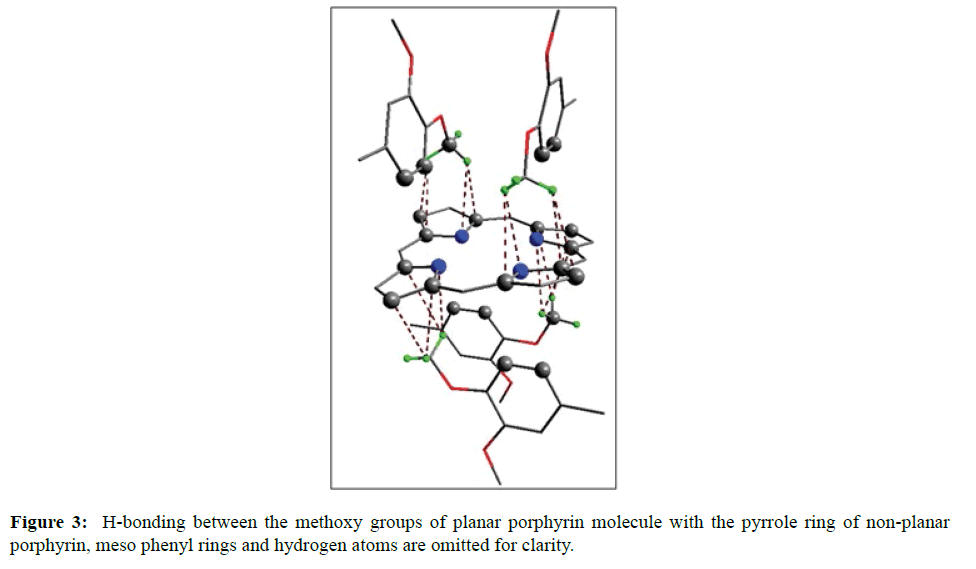
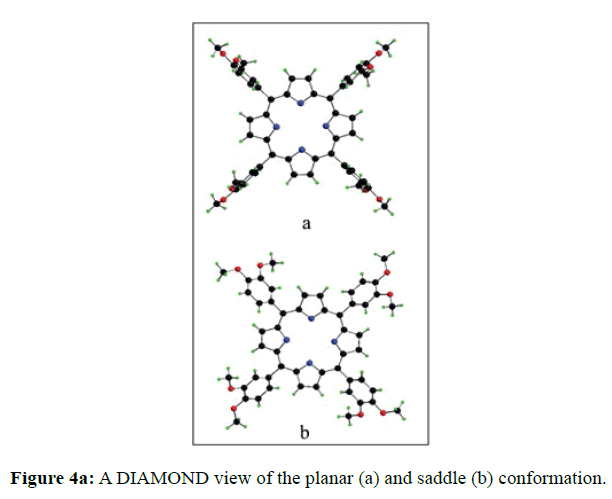
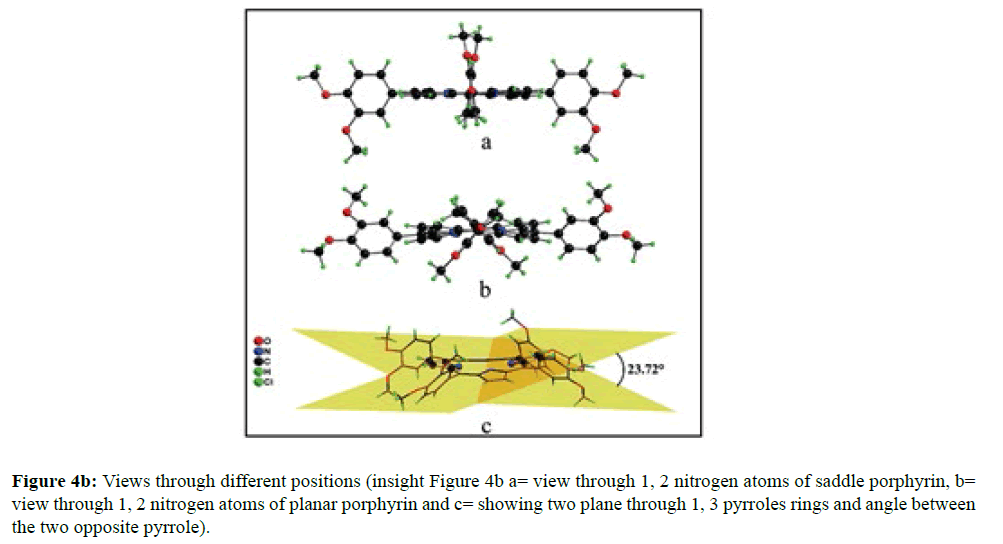
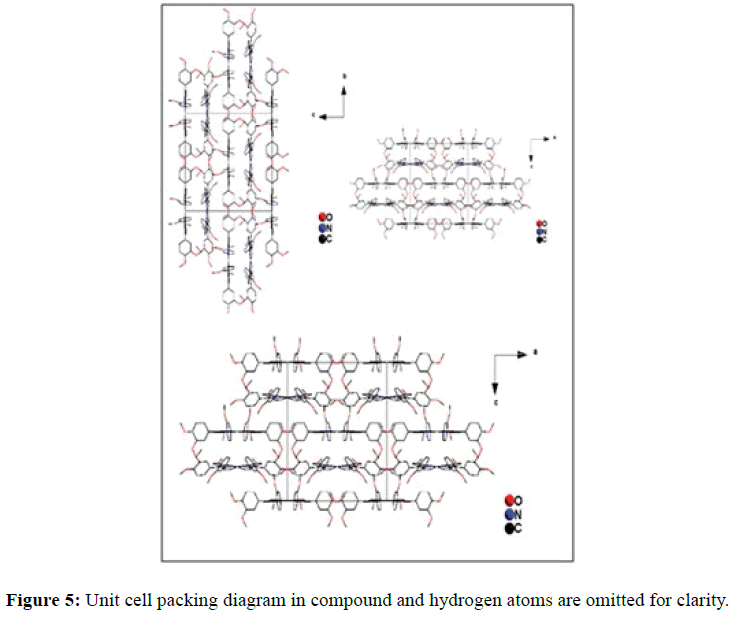
In the crystal structure of meso-tetrakis(3,4-dimethoxyphenyl)porphyrin, asymmetric unit contains ¼ of the two porphyrin molecule that is why we are unable to fix the hydrogen atom on the nitrogen of pyrrole ring. Out of the two molecule, one porphyrin molecule is almost planar and other is saddle in shape as compared with the structure of H2TPP [23]. The porphyrin core of the planar molecule consisting of 24 atoms which is non planar and the deviation from the mean plane does not exceed 0.1 Å but in the case of saddle molecule the deviation from the mean plane does not exceed 0.4 Å. The meso phenyl ring in the saddle porphyrin attached to the carbon atoms C17 are not perpendicular to the mean plane of the porphyrin ring and make a dihedral angle of 47.8ο with the core as compared to the angles 61.1 and 63.1ο in H2TPP. The torsion angles of C18-C17-C19-C24=126.2, C16-C17-C19-C24=52.9ο were observed in the saddle molecule. The meso phenyl groups in the saddle porphyrin are more tilted in the case of meso-tetrakis(3, 4-dimethoxyphenyl)porphyrin as compared to the H2TPP. But in the case of planar porphyrin the phenyl rings are almost planar and are oriented almost perpendicular to the porphyrin ring at an angle of 75.4-91.1ο as compared to that for meso-tetrakis(3,5-dihydroxyphenyl)porphyrin and meso-tetrakis(3,5-dinitrophenyl)porphyrin 56.0ο-68-5ο [24]. The observed bond lengths and bond angles of porphyrin macrocycles are not significantly different from those of H2TPP [23] as observed in the present case of meso-tetrakis(3,4-dimethoxyphenyl)porphyrin. The torsion angles of C1-C5-C6-C11=85.05 and C4-C5-C6-C7=84.84ο were observed. The meso phenyl group attached to the C5 carbon atom of the planar ring makes a dihedral angle of 82.7ο to the porphyrin core, a slight deviation occurs from the perpendicular position. Interesting feature observed in this porphyrin is the mixture of ruffled and planar molecule in the unit cell packed one after one. This may be explained on the basis of the fact observed in the structure that main cause for this type of behavior of this porphyrin is hydrogen bonding between the hydrogen of the methoxy group of planar molecule to the nitrogen as well as carbon of the pyrrole ring of another molecule which turns into the saddle shape which has been shown in the Figure 3. 1,3 pyrrole of the ring are making hydrogen bond from top side from the mean plane while 2,4 pyrrole make hydrogen bond with bottom side from the mean plane. This type of hydrogen bonding is C – H …….π type interaction. The distance between the C-H of methoxy group making hydrogen bond with π-orbital of the pyrrole is 2.96 Å. A DIAMOND view of planar and saddle conformation of free base porphyrin has been presented in the Figure 4a (insight Figure 4a, a= planar, b= saddle). The angle between the two opposite pyrrole formed by the plane passing through the pyrrole was found to be 23.72ο and the views through the different positions have been presented in the Figure 4b. The unit cell packing diagram is shown in the Figure 5 where hydrogen atoms are omitted for clarity. The porphyrins rings are arranged in one dimension only with CH – π interaction and π- π staking interaction among the porphyrin rings. The distance between the oxygen atoms of para methoxy group of planar molecule to that of two neighboring methoxy group of ruffled porphyrin in one dimensional array is 5.204 Å while that of oxygen atoms of meta position is 4.893 Å with the methoxy group of para positions. The intermolecular closest distance between the aryl group carbon and methoxy group oxygen is 2.38 Å. The distance between the nuclear least square plane of planar molecule and ruffled molecule is 4.627 Å.
Conclusion
In this paper, we have shown that crystal structure of 3,4-dimethoxyphenylporhyrin show unique hydrogen bonding interaction and have potential to be used in several porphyrin based materials in different applications.
References
- Suslick KS (1996), Comprehensive Supramolecular Reactivity and Transport: Bioinorganic Systems. Elsevier,USA.
- Robison GW, Brookhaven (1967), On the theory of trapping of excitation in the photosynthetic unit. Symp. Biol 19: 16-21.
- Yadav O, Varshney A, Kumar A (2017) Manganese(III) mediated synthesis of A2B Mn(III) corroles: A new general and green synthetic approach and characterization. Inorganic Chemistry Communications 86: 168–171.
- Fleming GR, Martin J L, Breton J (1988), Rate of primary electron transfer in photosynthetic reaction centers and their mechanistic applications Nature 333: 190-192.
- Paddock ML, Rongey SH, Feher G, Okamura MY (1989) Pathway of proton transfer in bacterial reaction centers: Replacement of glutamic acid 212 in the L subunit by glutamic inhibits quinone (secondary acceptor) turnover. Proc. Natl. Acad. Sci USA 86: 6602-6606.
- Feher G, Allen JP, Okamura MY, Rees DC (1989) Structure and function of bacterial photosynthetic reaction centres. Nature 339: 111-116.
- Holten D, Kirmarier C (1987) Primary photochemistry of reaction centers from photosynthetic purple bacteria. Photosynth Res 13: 225-260.
- Girolami GS, Hein CL, Suslick KS (1996), A Zirconium BisBis(porphyrin) Sandwich Complex with an Appended Quinone. Angew. Chem. Intl. Edn. 35: 1223-1225.
- Kong JL, Loach PA (1978) Model reactions in photosynthesis, Frontier Biol Energ I: 73-78.
- Neil MP, Niemczyk MP, Svec WA, Gosztola D,Gaines GL et al. (1992) Picosecond optical switching based on biphotonic excitation of an electron donor-acceptor-donor molecule. Science 257: 63-5.
- Wanger R, Lindsey JS (1994) A molecular photonic wire. J. Am. Chem. Soc 116: 9759-9760.
- Wanger R, Lindsey JS, Seth J, Palaniappan V, Bocian DF (1996) Molecular Optoelectronic Gates. J. Am. Chem. Soc. 118: 3996-3997.
- Shimidzu T (1995) Terminology for membranes and membrane processes, Pure Appl. Chem. 67: 11193.
- Shimidzu T, Iyoda T, Koide Y (1985) An advanced visible-light-induced water reduction with dye-sensitized semiconductor powder catalyst J. Am. Chem. Soc. 107: 35-41.
- Gau D, Li J, Ru- Qin Y, Zheng G (1978) Selective Extraction and Enrichment of Multiphosphorylated Peptides Using Polyarginine-Coated Diamond Nanoparticles. Anal. Chem. Soc. 100: 5075-5079.
- Yoo IJ, Shin JH, Paeng IR, Nam H, Cha GS et al. (1998) Current status of modern analytical luminescence methods. Anal. Chim. Acta 367: 175-178.
- Sessler JL, Kral V, Genge JW, Thomas RE, Iverson BL (1998) Anion Selectivity of a Sapphyrin-Modified Silica Gel HPLC Support, Anal. Chem. 70: 2516-2522.
- Malinski T, Ciszewski A, Fish JR, Czuchajowski L (1990) Conductive polymeric tetrakis(3-methoxy-4-hydroxyphenyl)porphyrin film electrode for trace determination of nickel. Anal. Chem. 62: 909-914.
- Gupta VK, Ajay KJ, Singh LP, Khurana U (1997) Porphyrins as carrier in PVC based membrane potentiometric sensors for Nickel (II), Anal. Chim. Acta. 355: 33-45.
- Dobson DJ, Saini S (1997), Porphyrin-Modified Electrodes as Biomimetic Sensors for the Determination of Organohalide Pollutants in Aqueous Samples. Anal. Chem 69: 3532-3538.
- Priyantha N, Weerabahu D (1996) Amperometric sensor for propanil. Anal. Chim. Acta. 320: 263-268.
- Kumar A, Maji S, Dubey P, Abhilash GJ, Pandey S, et al. (2007) One-pot General Synthesis of Metalloporphyrins. Tett. Lett 48: 7287-7290.
- Silvers SJ, Tulinsky A (1967), The crystal and molecular structure of triclinic tetraphenylporphyrin. J. Am. Chem. Soc. 89: 3331-3337.
- Bhyrappa P, Suslick, KS (1998) Synthesis and Crystal Structure of 5,10,15,20-Tetrakis (3,5-dinitrophenyl)porphyrin J. Porphyrins and Phthalocyanines 2: 391-396
- Vlascici D, Cosma E, Pica EM, Cosma V, Bizerea O, et al.(2008), Free Base Porphyrins as Ionophores for Heavy Metal Sensors Sensors (Basel). 8: 4995-5004.
- Fadda A, Mekawy RE, El-Shafei A, Freeman HS, Hinks D et al. (2012), Design, Synthesis, and Pharmacological Screening of Novel Porphyrin Derivatives. JOC. 2013: 11.
- Antonangelo AR, Westrup KCM, Burtb LA, Grazia BC, Malewschika T et al.(2017), Synthesis, crystallographic characterization and homogeneous catalytic activity of novel unsymmetric porphyrins. RSC Adv. 7: 50610-50618.
- Cosma EF, Vlascici D, Cosma GF, Palade A, Lascu A, et al. (2014), A Sensitive A3B Porphyrin Nanomaterial for CO2 Detection ,Molecules. 19: 21239-21252.
- Ding K, Yewei ZZ, Si W, Zhong X, Cai Y et al. (2018) Zinc(II) Metalated Porphyrins as Photothermogenic Photosensitizers for Cancer Photodynamic/Photothermal Synergistic Therapy. ACS Appl. Mater. Interfaces, 10: 238-247.
- Cosma EF, Badea V, Cosma GF, Palade A, Lascu A et al.(2017) Trace Oxygen Sensitive Material Based on Two Porphyrin Derivatives in a Heterodimeric Complex. Molecules. 22: 1787.
- Antonangelo AR, Westrup KCM, Burt LA, Grazia Bezzu C, Malewschik TGS et al. (2017) Synthesis, crystallographic characterization and homogeneous catalytic activity of novel unsymmetric porphyrins. RSC Adv.7: 50610-50618.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences