Synthesis and Characterization of Hybrid Compounds for the Treatment of Bone Related Diseases
Victor Nyarugwe*
University of Fort Hare, South Africa
- *Corresponding Author:
- Victor Nyarugwe
Department of Chemistry
University of Fort Hare, South Africa
Tel: +27-63-212-3467
E-mail: victornyarugwe@hotmail.com
Received Date: November 15, 2018; Accepted Date: December 11, 2018; Published Date: December 22, 2018
Citation: Nyarugwe V (2018) Synthesis and Characterization of Hybrid Compounds for the Treatment of Bone Related Diseases. J trauma Orth Nurs. 3:1.
Abstract
Bone diseases are major causes of abnormalities of the human skeletal system. To mention a few, osteoporosis, osteogenesis imperfecta and bone cancer are examples. This study is mainly focusing on osteoporosis and bone cancer. Osteoporosis have claimed many lives and some call it a silent thief because it has no clinical manifestations until there is a fracture. Bone cancer is also a life threating disease due to its effect of reducing red blood cell level. Bisphosphonates are well known antiresorptive drugs with the ability to inhibit the bone resorption process and diethylenetriamine compounds are chelating agents which can be used in chemotherapy to inhibit proliferation of several human cancers. So, the current research is aiming to make one more step in chemistry of hybrid compounds with high potency, high efficacy and low toxicity. All the reactions were performed at room temperature then, infrared and nuclear magnetic resonance were used to characterize the synthesized compounds.
Keywords
Bone diseases; Osteoporosis; Antiresorptive drugs; Chemotherapy; Diethylenetriamine compounds
Introduction
The living bone is a porous structure of fluid and living cells in a constant state of flux. In a normal health organism, the bone undergoes a continuous renewal process [1] where, senescent bones are constantly being removed by bone-resorbing cells known as osteoclasts and being replaced by new bone-formation cells called osteoblasts [2]. During childhood age, the body is capable of adding new bone faster than it removes the old one. Normally after the age of 20 the reverse process starts to occur, where one can lose bone faster than he/she can make it. When it comes to building strong bones they are two key nutrients which are calcium and vitamin D, calcium maintain bone rigidity and teeth structure, while vitamin D improves intestinal absorption of calcium and bone growth [3]. However, these nutrients are important early in life but they may also help as you age, also exercises are required [4].
Types of bone diseases
Different kinds of bone diseases include osteoporosis, cancer and osteogenesis imperfecta, osteomyelitis and osteosarcoma.
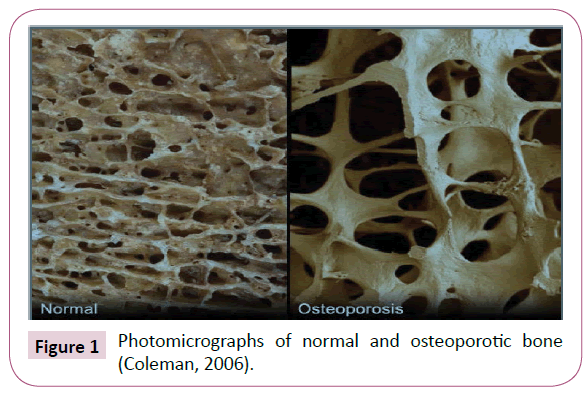
Osteoporosis: Is a disease whereby an imbalance in the bone remodelling process result in more bone removal than replacement and this leads to an increase in the risk of fracture [5]. Figure 1 shows the changes with bone as a result of bone loss.
Bone cancer: Bone cancer is another type of bone disease and it can begin in any bone but, it is commonly found in long bones such as those in the legs, arms or pelvis. However, bone tumours are very rare and they are estimated to be less than one percent of all cancers [6]. People suffering from bone cancer show the following signs and symptoms [7].
• Bone pain
• Swelling and tenderness near the affected area
• Weakened bone, leading to fracture
• Fatigue
• Unintended weight loss
Risk factors
It's not clear what causes bone tumour, but certain factors have been found to be associated with an increased risk, including [8].
1. Paget's disease of bone: interferes with your body's normal recycling process
2. Radiation therapy for cancer: exposure to large doses of radiation, such as those given during radiation therapy for cancer, increases the risk of bone cancer in the future.
3. Genetic inherited syndromes: such as retinoblastoma On the other hand, risk factors of osteoporosis include [9].
1. Age, normally people who are at the age of 65 and above have a greater risk
2. Gender, women are at greater risk than men due to menopause
3. Also family history plays a major role for example if any of your ancestors suffered from this disease there will be a probability that one or more of their descendants might suffer from it.
4. Smoking and drinking too much alcohol
Current concepts in the treatment of bone diseases
There has been significant progress in bone disease treatment, bisphosphonates drugs are commonly used class of medicines to treat osteoporosis. These include alendronate, zoledronic acid and risendronate [10].
The most commonly used drugs for bone cancer treatment include cisplatin, vincristine doxorubicin and etoposide. However, most of these medications today are known to pose some side effects such as nausea, heartburn, stomach pains and hair loss. Also, chemotherapy can damage the blood-producing cells in the bone marrow, so you may have low blood cell counts (American Cancer Society, 2018).
Literature Review
In the past, people thought a shortage of estrogen caused bone diseases. People thought this because most patients were women who came down with osteoporosis after menopause [11]. According to World Health Organization records, bone diseases are recognized as a major public health problem in both developing and developed countries. Since this is an alarming issue in the society today, the most reasonable thing the world should do is to try to find a way that prevents the bone from losing its density. However, this became the major area of interest for researchers since osteoporosis was officially acknowledged and defined as a disease by the World Health Organization in 1994 [12]. The development of bisphosphonate drugs began when trace amounts of polyphosphonates were shown to be capable of inhibiting the bone resorption process.
Several members of bisphosphonates are either in clinical use or well advanced in clinical trials. However, the main focus is on the differences in potency, route of administration, duration of action, and efficacy between members of this expanding class of drugs. The initial observations that the bisphosphonate structure had high affinity for bone and inhibits the degradation of hydroxyapatite crystals led to confirmation that they could inhibit bone resorption in vitro [13].
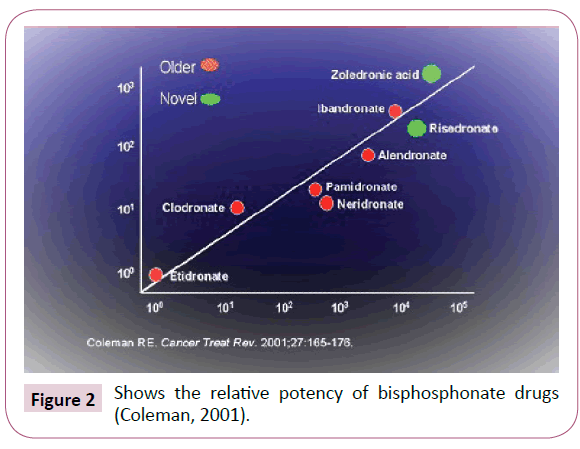
Despite the great potential of bisphosphonates drugs in bone disease treatment some researchers found out that the potency of bisphosphonates can be enhanced by manipulation of the composition of the side-chains attached to the P-C-P core [14]. Of the currently available bisphosphonates, etidronate and clodronate are non-Nitrogen (N)- containing-bisphosphonates (which do not contain nitrogen atoms in their side-chains), whose antiresorptive potency is at the lower end of the scale, while pamidronate, alendronate, risedronate, Ibandronate, and zoledronate are N-containing-bisphosphonates which exhibit antiresorptive potencies 100-10,000-fold that of etidronate as shown in the Figure 2 [15].
Such results were derived from experiment involving nitrogen containing (NBPs) bisphosphonates as well as non-nitrogen-containing bisphosphonates (NNBPs) in dose response studies. It has been shown that NNBPs affect osteoclasts mainly through apoptosis whereas NBPs are able to exhibit the target organ without inducing apoptosis [16]. This is explaining why NBPs can work more efficiently even at low concentration (high potency) [17].
According to [18], bisphosphonates bind to the surfaces of the bones and slow down the bone resorping action of the osteoclasts (bone-eroding cells). This allows the osteoblasts (bone-building cells) to work more effectively. Structurally, bisphosphonates are chemically stable derivatives of inorganic pyrophosphate (PPi), a naturally occurring compound in which two phosphate groups are linked by esterification [19].
Pioneering studies from the 1960s demonstrated that PPi was capable of hindering calcification by binding to hydroxyapatite crystals, leading to the assumption that regulation of PPi levels could be the mechanism by which bone mineralization is regulated. Almost all bisphosphonates in current clinical use also have a hydroxyl group attached to the central carbon (termed the R1 position) [20]. The flanking phosphate groups provide bisphosphonates with a strong affinity for hydroxyapatite crystals in bone (and are also seen in PPi), whereas the hydroxyl motif further increases a bisphosphonate’s ability to bind calcium.
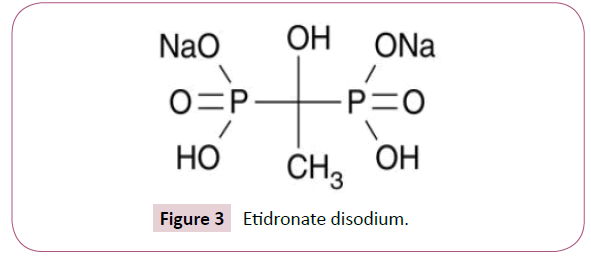
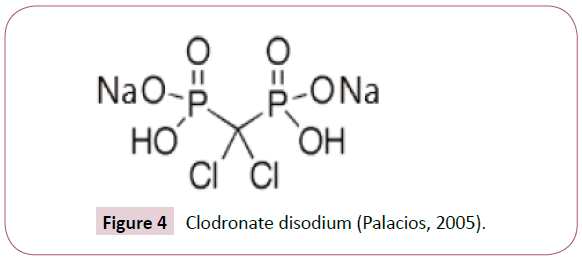
Although the phosphate and hydroxyl groups are essential for bisphosphonate affinity for bone matrix, the final structural moiety (in the R2 position) bound to the central carbon is the primary determinant of a bisphosphonate’s potency for inhibition of bone resorption. The presence of a nitrogen or amino group increases the bisphosphonate’s antiresorptive potency by 10 to 10,000 relative to early non-nitrogen-containing bisphosphonates, such as etidronate. Figures 3 and 4 are some of the first generation of bisphosphonates drugs which were used during early studies.
The diversity of these drugs is due to the lateral chains on the meso-atom of carbon, they have been classified as N-containing or non-N-containing. Early bisphosphonates are placed in the class of non-N-containing as they do not possess a nitrogen atom on the lateral chain [21].
Problem statement
According to International Osteoporosis Foundation (IOF) records, around the world an osteoporotic fracture is estimated to occur in every 3 seconds (IOF, 2018). It is therefore regarded more as a risk factor of bone fracture than a disease. Bone tumors remain a major challenge, this challenge is even heightened in developing countries due to limited diagnostic and therapeutic facilities as well as due to ignorance [22].
Of the present drugs, cispatin and zoledronic acid have been found to cause some undesirable side effects such as hair loss and renal toxicity [23]. Also Drake et al., [24] found out that the antiresorptive potency of N-containing bisphosphonates can be enhanced by manipulation of their side chains such as the synthesis of hybrid compounds.
Motivation and rationale
Nitrogen-containing BPs (N-BPs) have high potency as they are used at very low doses and are specifically targeted to osteoclasts and for that reason alendronate will be ideal to work with as it possesses a nitrogen atom. Case-control studies have shown circulating levels of vitamin D to be significantly lower in patients with bone diseases as compared to healthy controls, vitamin D is very important as it promotes intestinal absorption of calcium which is an element for healthy bones [25]. As a result, alendronate and vitamin D hybrids are favoured because of their increased ability for suppressing bone diseases at lower doses and less toxicity.
Aim
To synthesize hybrid compounds for the treatment of bone diseases using one-pot synthesis strategy followed by their characterization.
Objectives
1. To synthesize bisphosphonate amide, esters of vitamin D, vitamin D amides.
2. To characterize the synthesized compounds using Fouriertransformed infrared spectroscopy (FTIR) and nuclear magnetic resonance (NMR).
Materials and Method
This section gives the details of the materials and reactions to be followed in the synthesis of hybrid compounds.
Materials
The following chemicals and materials were used; 4-aminosalicyclic acid (C7H7NO3), cinnamic acid (C9H8O2), Diethylenetriamine (DET), Vitamin D2 (ergocalciferol), succinic anhydride(C4H4O3), Alendronate (C4H13NO7P2), distilled water, condenser, measuring cylinder, round bottomed flask, heating mantle and a stand, magnetic stirrer, acetone, N,N'- dicyclohexylcarbodiimide, dichloromethane, dimethylsulfoxide ,potassium tetrachloroplatinate(II), methanol all these reagents where bought from Sigma Aldrich, South Africa with an exception of alendronate which was already prepared and I received it from my supervisor.
Method
Esterification reaction: Esters were prepared using Steglich esterification method, the reason for not using direct esterification method is due to the OH functional group attached to the ring which may result in steric hindrance. In this method dicyclohexylcarbodiimide (DCC) will be serve as a as a coupling reagent in other words acts as a link joining two bulk groups together, 4- Dimethylaminopyridine (DMAP) serving as a catalyst and dimethylsulfoxide (DMSO) or dichloromethane (DCM) as a solvent where the reaction will take place.
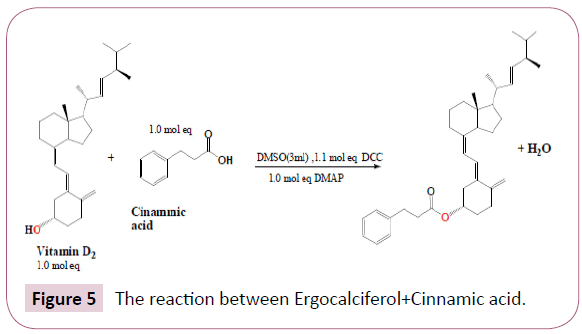
Ergocalciferol+Cinnamic acid: Cinnamic acid of 1.0 mol equivalent to the 100 mg of ergocalciferol was added to 3 ml of DMSO in a round bottomed flask followed by 1.1mol equivalent of DCC then 1.0 mol equivalent of vitamin D2. DMAP of 1.0 equivalent was also added to the mixture, the resulting mixture was stirred using a stirrer bar, stirring is important as this will speed up the reaction rate according to the collision theory this will increase the number of collisions between reactant molecules. This reaction was left for overnight at room temperature. The Table 1 summarizes this procedure. The reaction occurred as in Figure 5.
| Substance | Quantity (g) | Formula weight (g/mol) | Moles | mol. equivalent |
|---|---|---|---|---|
| Ergocalciferol | 0.1 | 396.659 | 0.0002520 | 1.0 |
| DCC | 0.05719 | 206.33 | 0.0002772 | 1.1 |
| DMAP | 0.03079 | 122.17 | 0.0002520 | 1.0 |
| Cinnamic acid | 0.03733 | 148.16 | 0.0002520 | 1.0 |
| DMSO | 3ml |
Table 1: Ergocalciferol+Cinnamic acid.
On the following day, the progress of the reaction was checked by spotting the contents of the flask on a TLC plate against its initial reactants, this was done to check if all reactants were completely used up. The results displayed on the TLC plate are given in the results section below. After that, filtration was done in order to remove DMAP which was observed as sediments in the flask. After that process cold work-up was performed using ice water and dichloromethane (DCM).The main purpose of an aqueous work-up is to isolate and purify the product. Since reaction product was soluble in the organic phase this means that any other impurities which are water soluble would remain in the aqueous phase. The resulting organic phase was evaporated in the hood, the remaining product was kept in the sample vial for further analysis.
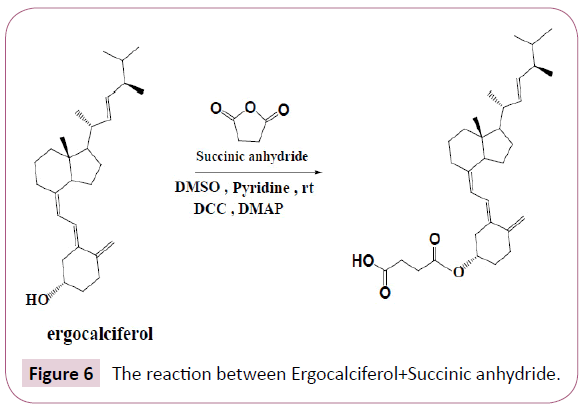
Ergocalciferol+Succinic anhydride: Ergocalciferol was weighed and placed in a round bottomed flask followed by succinic anhydride then DMAP, DCC, pyridine and DMSO. The reaction was stirred for overnight at room temperature. The Table 2 shows the quantities of the reactants used. The reaction occurs as Figure 6.
| Substance | Quantity (g) | Formula weight (g/mol) | Moles | mol. equivalent |
|---|---|---|---|---|
| Ergocalciferol | 0.1 | 396.659 | 0.0002520 | 1.0 |
| DCC | 0.05719 | 206.33 | 0.0002772 | 1.1 |
| DMAP | 0.01539 | 122.17 | 0.0001260 | 0.5 |
| Succinic anhydride | 0.02520 | 100.07 | 0.0002520 | 1.0 |
| DMSO | 3ml | |||
| Pyridine | 1ml |
Table 2: Ergocalciferol+Succinic anhydride.
On the following day, the progress of the reaction was checked by spotting the contents of the flask on a TLC plate against its initial reactants, this was done to check if all reactants were completely used up. The results displayed on the TLC plate are given in the results section below. After that, filtration was done in order to remove DMAP which was observed as sediments in the flask. After that process an aqueous work-up was performed using distilled water and dichloromethane (DCM).The main purpose of an aqueous work-up is to isolate and purify the product. Since reaction product was soluble in the organic phase this means that any other impurities which are water soluble would remain in the aqueous phase. The resulting organic phase was evaporated in the hood, the remaining product was kept in the sample vial for further analysis.
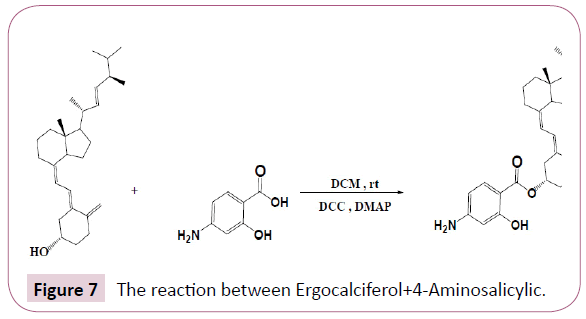
Ergocalciferol+4-Aminosalicylic: Ergocalciferol was weighed and placed in a round bottomed flask followed by 4-aminosalicylic then DMAP, DCC, and DCM as a solvent. The reaction was stirred for overnight at room temperature. The Table 3 shows the quantities of the reactants used. The reaction occurs as in Figure 7.
| Substance | Quantity (g) | Formula weight (g/mol) | Moles | mol. equivalent |
|---|---|---|---|---|
| Ergocalciferol | 0.1 | 396.659 | 0.0002520 | 1.0 |
| DCC | 0.05719 | 206.33 | 0.0002772 | 1.1 |
| DMAP | 0.01539 | 122.17 | 0.0001260 | 0.5 |
| 4-aminosalisacylic | 0.03859 | 153.135 | 0.0002520 | 1.0 |
| DCM | 5 ml |
Table 3: Ergocalciferol+4-Aminosalicylic.
The progress of the reaction was checked by spotting the contents of the flask on a TLC plate against its initial reactants, this was done to check if all reactants were completely used up. The results displayed on the TLC plate are given in the results section below and Rf values were calculated.
Amidation reaction
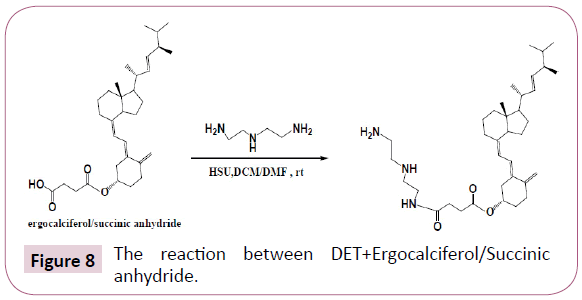
DET+Ergocalciferol/Succinic anhydride: Ergocalciferol/ Succinic anhydride product formed was reacted with diethylenetriamine (DET) using DCC as a coupling reagent and DMAP as a catalyst, this reaction was performed at room temperature. The Table 4 shows the quantities used. The reaction occurred as in Figure 8.
| Substance | Quantity (g) | Formula weight (g/mol) | moles | mol. equivalent |
|---|---|---|---|---|
| Ergocalciferol/Succinic anhydride | 0.15 | 492.659 | 0.00030447 | 1.0 |
| DCC | 0.0691 | 206.33 | 0.00033492 | 1.1 |
| HSU | 0.0350 | 115.09 | 0.00030447 | 1.0 |
| DET ( 0.033ml) | 0.0314 | 103.17 | 0.00030447 | 1.0 |
| DCM/DMF | 3:1 respectively |
Table 4: DET+Ergocalciferol/ Succinic anhydride.
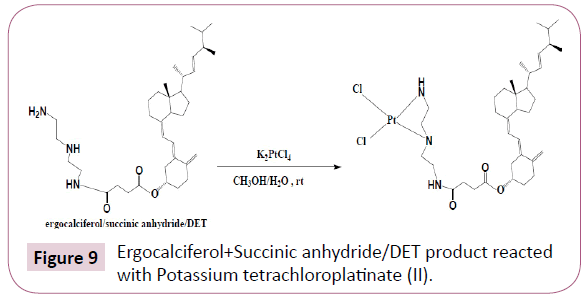
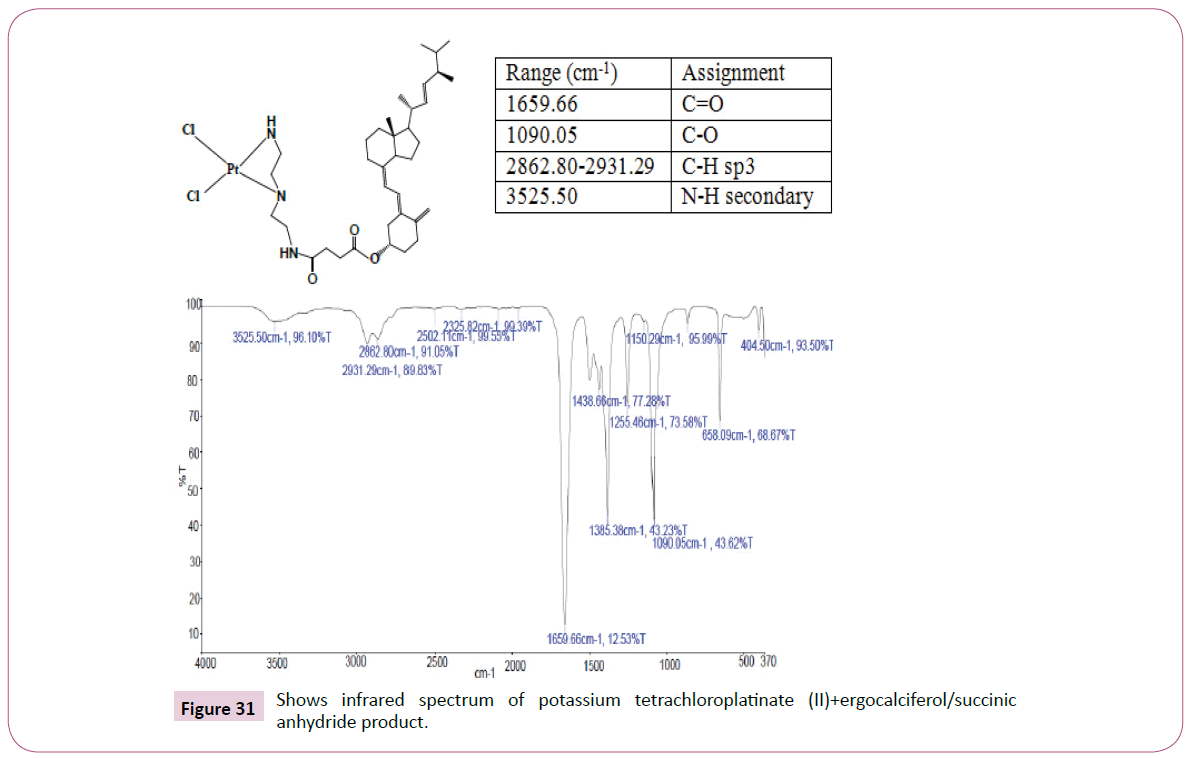
Then the ergocalciferol/Succinic anhydride/DET product formed was then reacted further with Potassium tetrachloroplatinate (II). This reaction was performed at room temperature for 24 h, the contents of the reaction flask were concentrated using the rotary evaporator. The product formed was filtered and the filtrate was freeze dried, also the residue was kept for further analysis (Table 5). The reaction occurred as in Figure 9.
| Substance | Quantity(g) | Formula weight (g/mol) | Moles | mol equivalent |
|---|---|---|---|---|
| Ergo/succ/DET | 0.1429 | 577.829 | 0.0002473 | 1.0 |
| K2PtCl4 | 0.1027 | 415.09 | 1.0 | |
| Methanol/water | 5ml |
Table 5: Ergocalciferol/Succinic anhydride/DET product reacted with Potassium tetrachloroplatinate(II).
Methanol and water mixture was used as a solvent system, simply because the ergocalciferol/succinic acid/DET product was soluble in methanol but sparingly soluble in water, on the other hand tetrachloroplatinate (II) is insoluble in methanol but soluble water, so water/methanol was the best solvent system to use. Also the other reason is that water and methanol are miscible. This reaction was not spotted on the TLC plate as inorganic compounds are UV-Visible inactive.
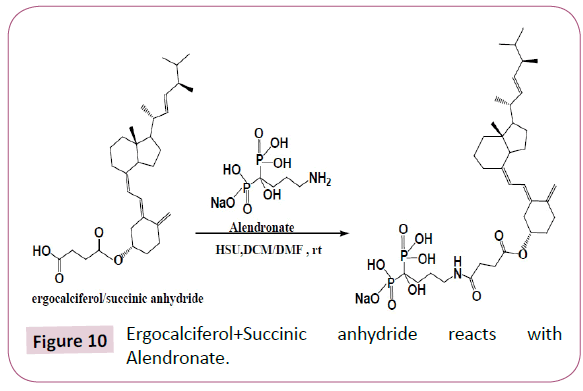
Ergocalciferol/Succinic anhydride+Alendronate
Ergocalciferol/Succinic anhydride product formed was then reacted with alendronate using DCM and DMF as solvents and HSU as a catalyst. The Table 6 shows the quantities of reagents used. The reaction occurred as in Figure 10.
| Substance | Quantity (g) | Formula weight (g/mol) | Moles | mol. equivalent |
|---|---|---|---|---|
| ergo/Succinic anhydride | 0.1320 | 492.659 | 0.00026793 | 1.0 |
| DCC | 0.0608 | 206.33 | 0.00029472 | 1.1 |
| HSU | 0.0308 | 115.09 | 0.00026793 | 1.0 |
| Alendronate | 0.0667 | 249.096 | 0.00026793 | 1.0 |
| DCM/DMF | 3:1 respectively |
Table 6: Ergocalciferol/ Succinic anhydride +Alendronate.
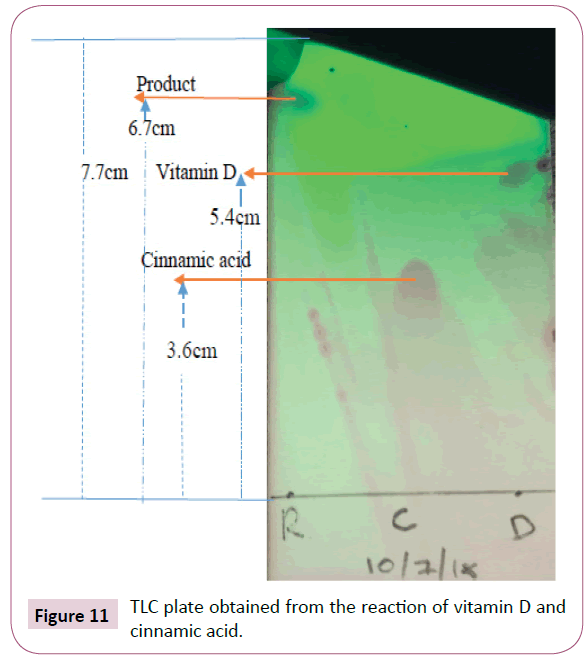
Thin layer chromatography was used to monitor the progress of the reaction and the TLC plate obtained from the reaction of vitamin D and cinnamic acid as shown in Figure 11, the solvent system used for the TLC was toluene, chloroform and methanol, the ratio was 1:6:1 respectively.
The distance moved by solvent front is 7.7cm
Rf value of the product=6.7cm/7.7cm= 0.87
Rf value of cinnamic acid=3.6cm/7.7cm= 0.47
Rf value of the vitamin D=5.4cm/7.7cm=0.70
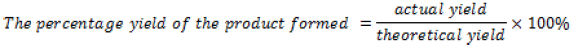
Actual yield=mass of cinnamic acid+mass of vitamin D=0.03733g+0.1g=0.13733g
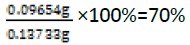
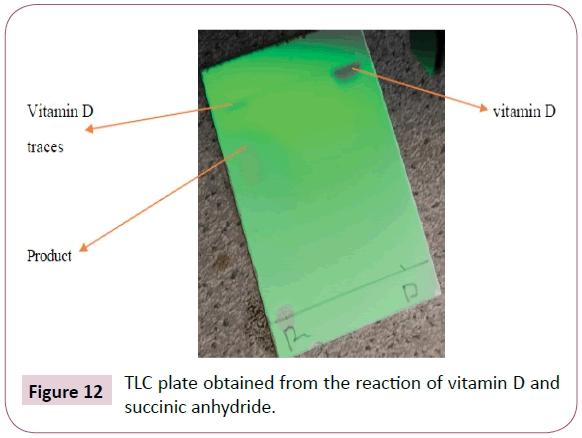
For the reaction of succinic anhydride and vitamin D (Figure 12)
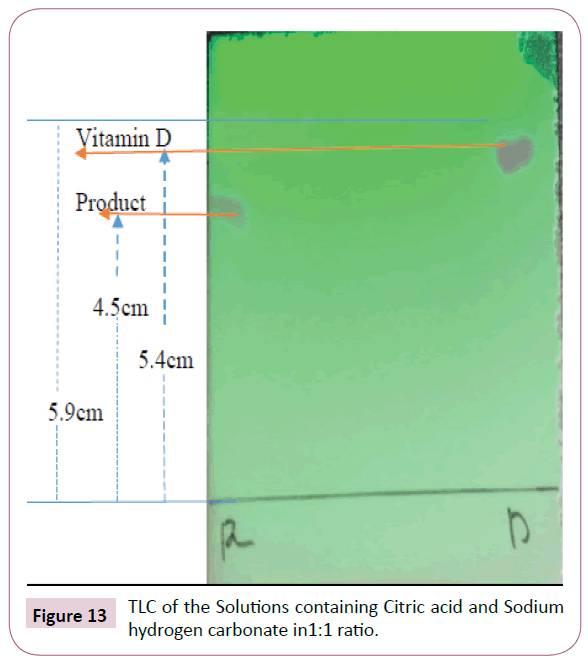
From the TLC plate above it can be observed that some traces of vitamin D were still visible after 24 hours, this is as a result of succinic anhydride being completely used up. More succinic anhydride was added to this reaction but nothing changed, the results were still the same. Succinic anhydride is not spotted as it does not appear under the UV light or spraying reagent. In an attempt to solve the problem above an acid work-up was carried out using 0.5M citric acid in 250ml and 0.5M sodium hydrogen carbonate. These solutions were prepared as follows
Citric acid:
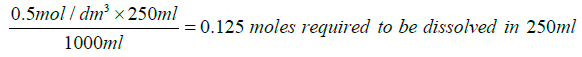
Mass to be dissolved=210.14 g/mol × 0.125 moles=26.2675 g
Sodium hydrogen carbonate:
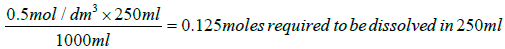
Mass to be dissolved=84.01 g/mol × 0.125 moles=10.50125 g
The prepared solutions were mixed in a ratio of 1:1 using DCM as an organic phase and the resulted TLC plate is as shown in Figure 13
The distance moved by solvent front is 5.9 cm
Rf value of the product=4.5 cm/5.9 cm=0.76
Rf value of the vitamin D=5.4 cm/5.9 cm=0.92
The percentage yield of the product formed 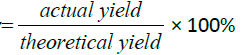
Actual yield=mass of succinic anhydride+mass of vitamin D=0.02520 g+ 0.1 g=0.1252 g
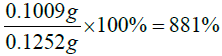
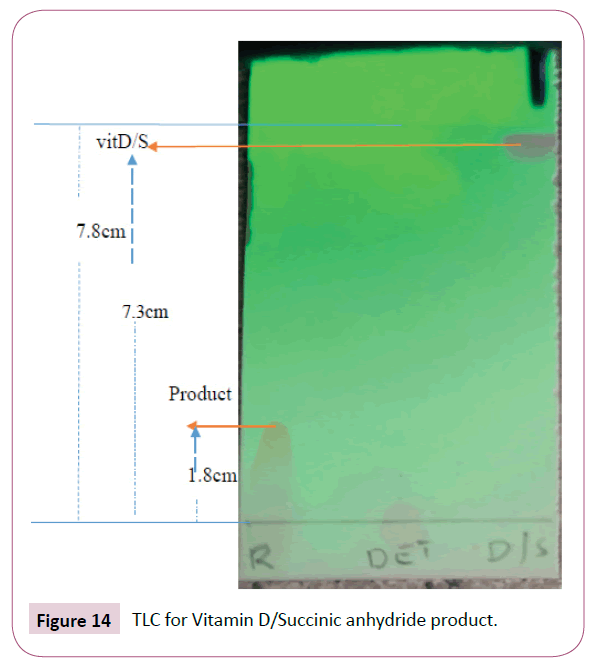
For the reaction of Vitamin D/ Succinic anhydride product formed above and DET
The TLC plate obtained is given below, DET does not appear under UV light as it is an inorganic compound (UV inactive). On this reaction, the solvent system of toluene, chloroform and methanol were not giving good resolution. Ethyl acetate, hexane and methanol in the ratio of 7:3:0.5 gave a better resolution (Figure 14).
The distance moved by solvent front is 7.8 cm
Rf value of the product=1.8 cm/7.8 cm=0.23
Rf value of the vitamin D=7.3 cm/7.8 cm=0.94
The percentage yield of the product formed 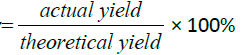
Actual yield=mass of DET+mass of vitamin D=0.0314 g+0.15 g=0.1814 g
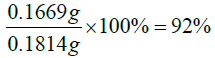
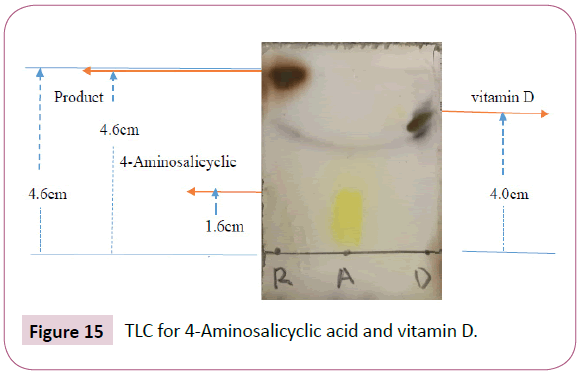
For the reaction of 4-Aminosalicyclic acid and vitamin D
The TLC plate obtained is shown in Figure 9.
The product formed in the reaction above was not visible under UV lamp, so the TLC plate was dipped in a ninhydrin spraying reagent and the spots started to appear after warming the plate in an oven (Figure 15).
The distance moved by solvent front is 4.6 cm
Rf value of the product=4.6 cm/4.6 cm=1
Rf value of the vitamin D=4.0 cm/4.6 cm=0.87
Rf value of the 4-Aminosalicyclic=1.6 cm/4.6 cm=0.35
The percentage yield of the product formed 
Actual yield=mass of 4-Aminosalisyclic+mass of vitamin D=0.031859 g+0.1 g=0.131859 g
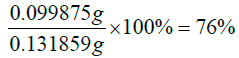
For the reaction of alendronate with ergocalciferol/succinic anhydride product and tetrachloroplatinate(II) with DET/ ergocalciferol/succinic anhydride product nothing was observed on TLC plates and this could be as a result of being UV inactive since both tetrachloroplatinate(II) and DET are inorganic compounds.
Characterization
Fourier transform infrared spectroscopy: Infrared Spectroscopy is the analysis of infrared light interacting with a molecule. This can be analyzed in three ways by measuring absorption, emission and reflection. This technique is mainly used by chemists to determine functional groups in molecules. This technique was performed on the hybrid compounds on Perkin Elmer Spectrum at a frequency of 100 MHz.
Nuclear magnetic resonance: Nuclear magnetic resonance (NMR) is a spectroscopic technique well suited for determining the structure of molecules ranging from a few atoms to large molecules. The strength of NMR lies in its ability to distinguish and identify atoms on the basis of their chemical environment, and in determining the chemical bond and spatial relationship of those atoms. Thus, NMR is a powerful tool for the identification of molecules, especially those of an organic nature. So this technique this technique was performed on hybrid compounds to determine the number of protons and carbons present on them. It was performed on Varian Unity INOVA 300 MHz.
Results and Discussion
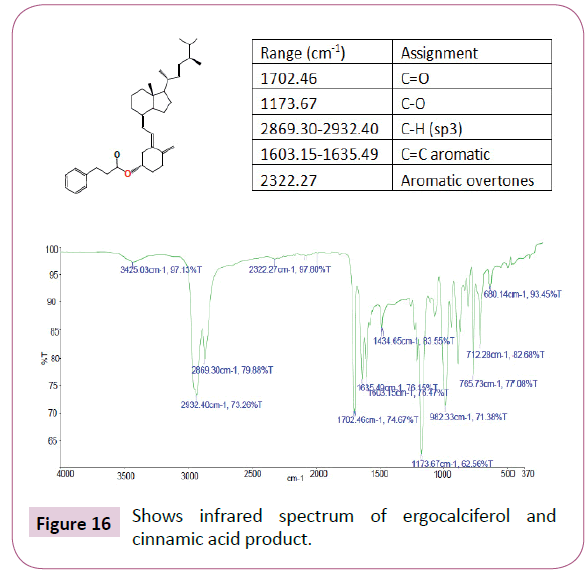
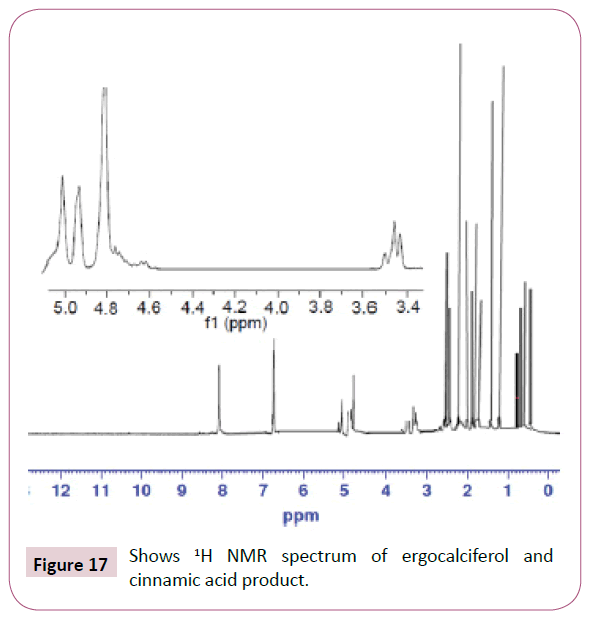
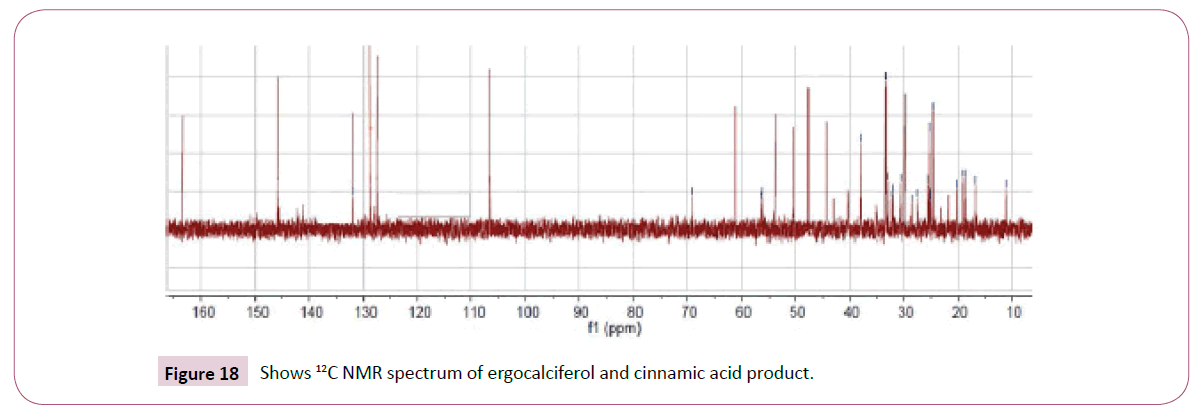
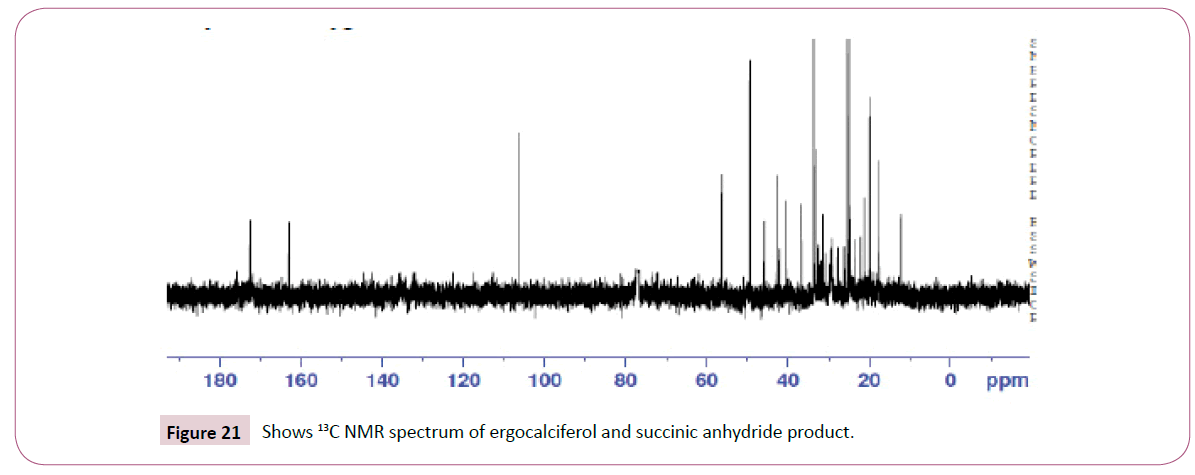
The peaks from 6.7-8 ppm are for protons in the benzene ring. The triplet peaks from 4.7-5 ppm are for protons on the carbon atom next to C=O since the neighbouring carbon has two protons. Another triplet at 3.5 ppm is for protons on the carbon atom close to the benzene ring which has also two neighbouring protons. The rest of the peaks upfield are for the protons attached to the carbon atom far away from the oxygen atom (Figures 16-18). Four peaks from 138 ppm to around 155 ppm represents four carbons of the benzene ring in different chemical environments. Peak at around 165 ppm represents the carbon atom of the ester. Peak at around 107 ppm represents the carbon atom bonded to the oxygen atom. The rest of the peaks upfield are from the alkane chain part of ergocalciferol [26-34].
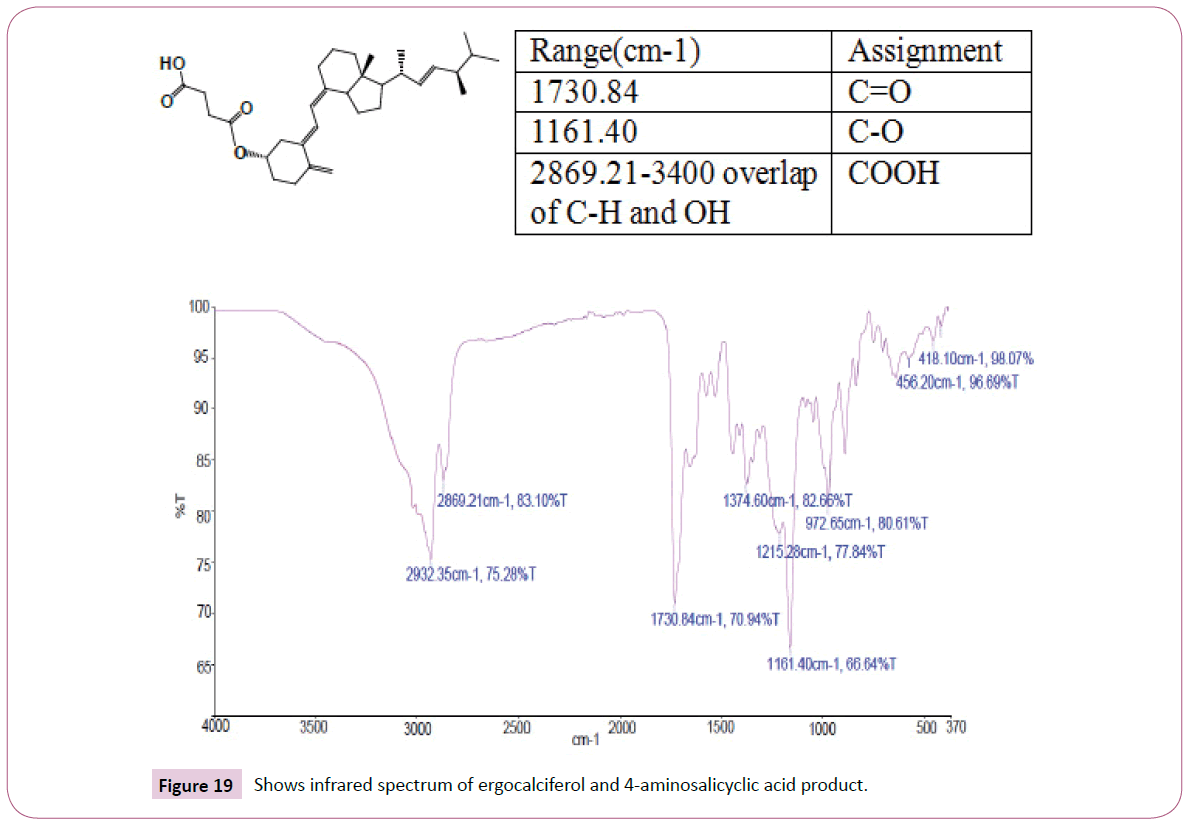
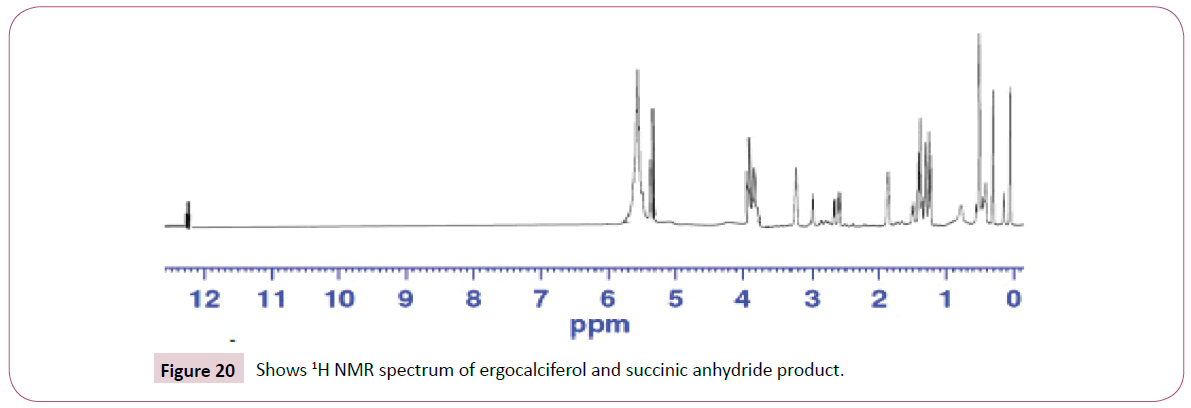
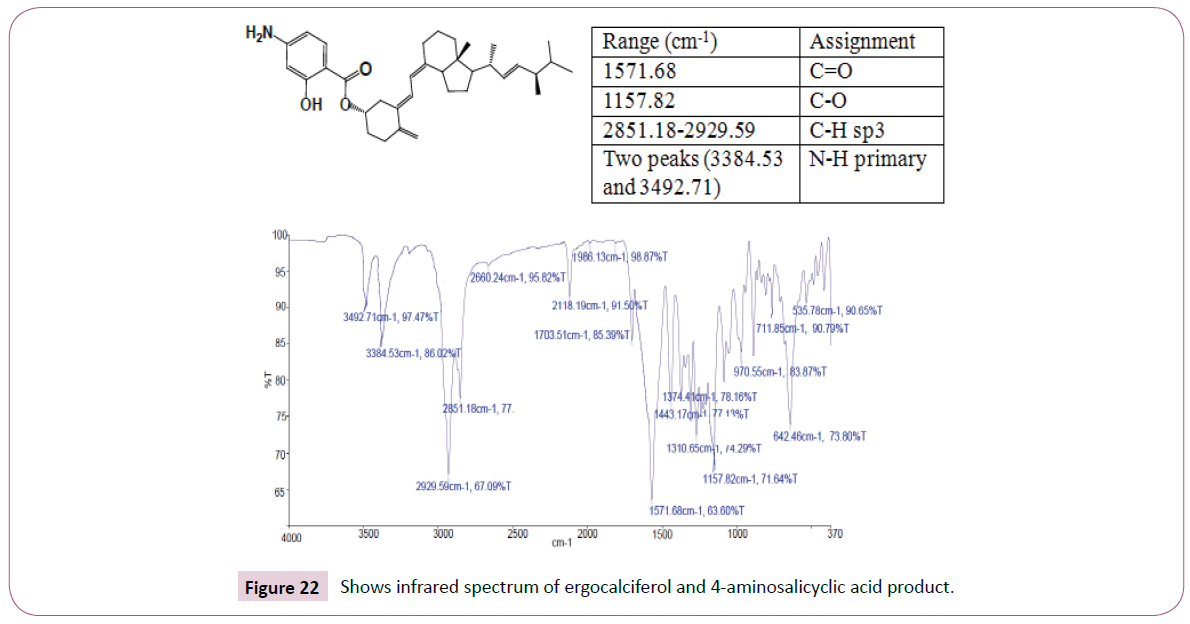
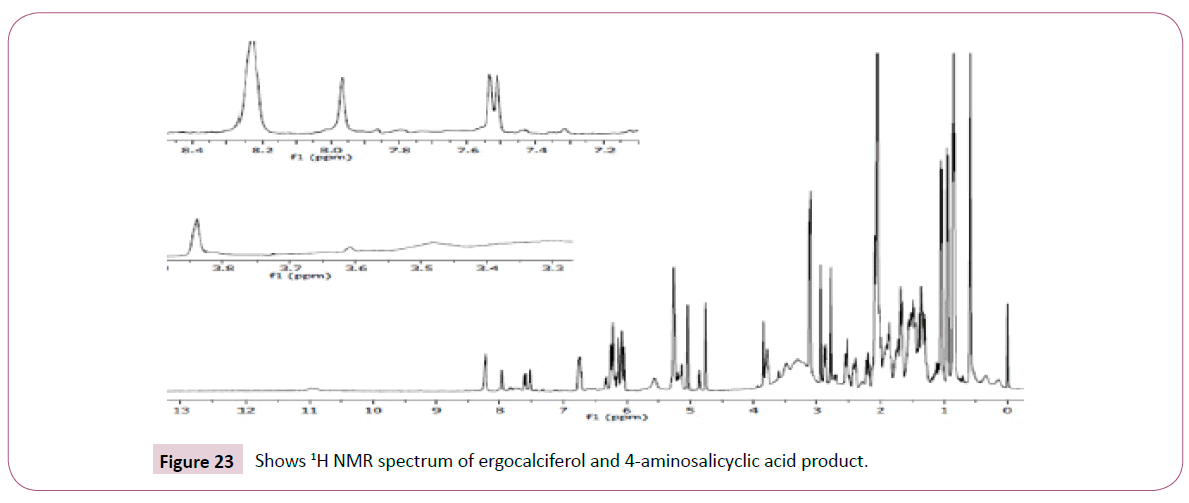
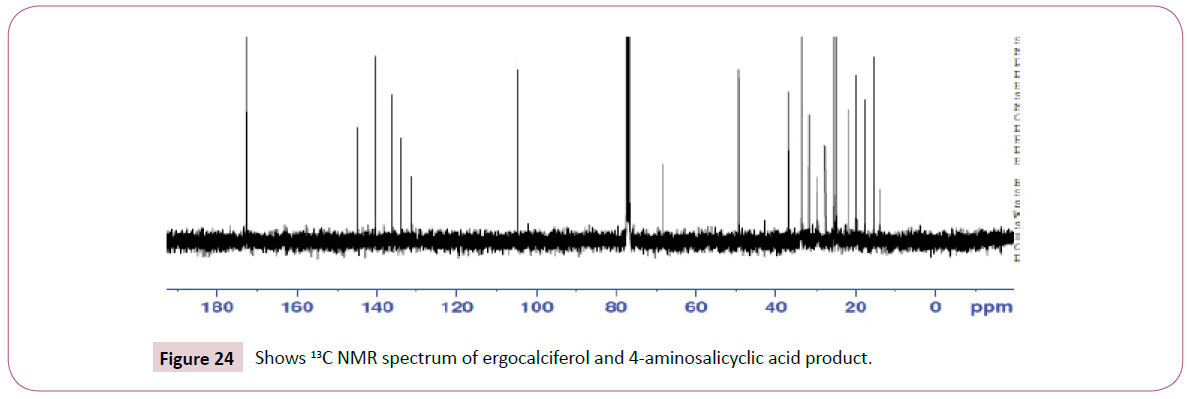
The singlet at 12 ppm represents the OH of the carboxylic acid. The triplet peaks from 5.2-5.7 ppm are for protons on the carbon atom next to C=O since the neighbouring carbon has two protons. Another triplet at 3.8 ppm is for protons on the carbon atom of the cyclohexane ring which has also two neighbouring protons and is also close to the carbon atom bonded to the oxygen atom. The rest of the peaks upfield are for the protons attached to the carbon atom far away from the oxygen atom (Figures 19-23). The peak at around 173 ppm represents the carbon atom of the carboxylic acid end. Peak at around 163 ppm represents the carbon atom of the ester. Peak at around 105 ppm represents the carbon atom bonded to the oxygen atom. The rest of the peaks upfield are from the alkane chain part of ergocalciferol. The peak at around 8.2 ppm represents the proton of the NH2 group. Peaks from 7.5-8.0 ppm represents the aromatic protons, then the singlet at 4.0 for the OH. The rest of the peaks upfield are for the protons attached to the carbon atom far away from the oxygen atom.
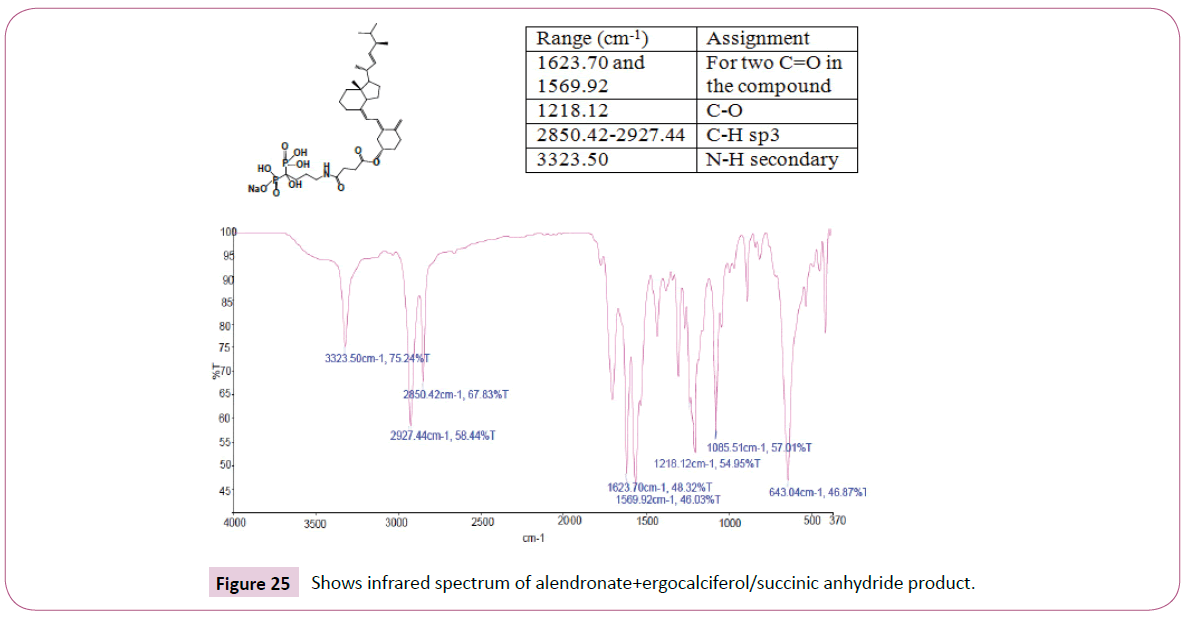
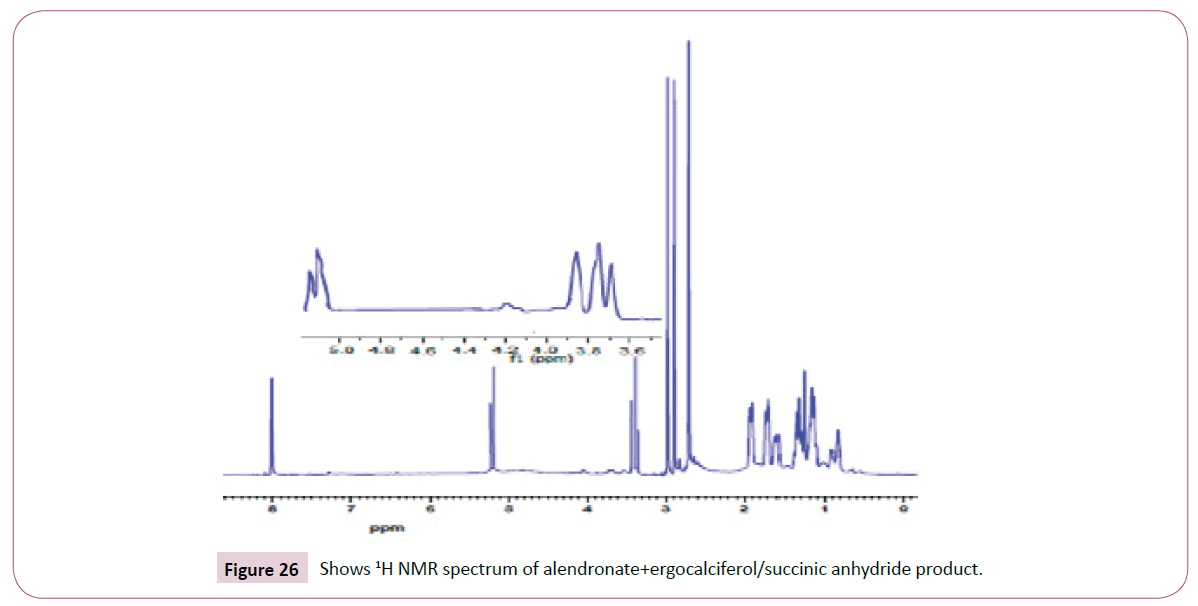
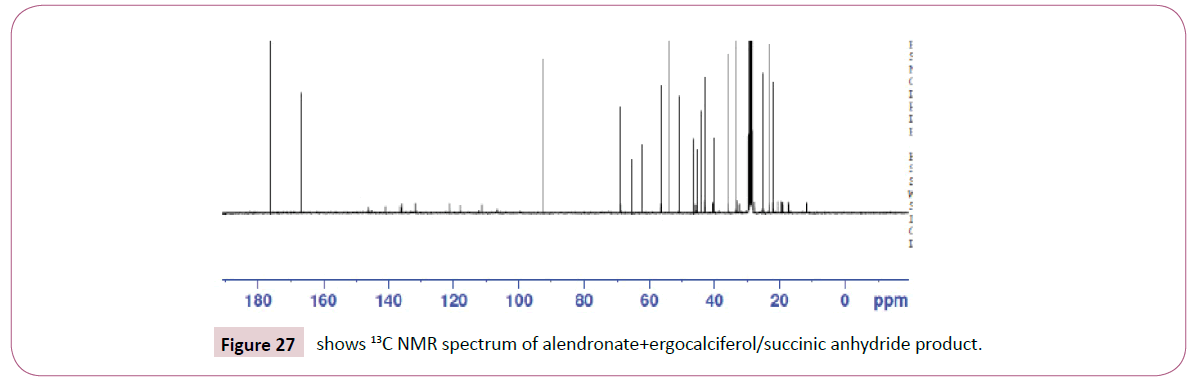
Peak at around 163 ppm represents the carbon atom of the ester. Five peaks between 130 ppm-155 ppm represents the carbon atoms of the aromatic ring. Peak at around 105 ppm represents the carbon atom bonded to the oxygen atom. The rest of the peaks upfield are from the alkane chain part of ergocalciferol (Figures 24-26). The peak at around 8.0 ppm represents the proton of the NH group. Doublet at 5.3 ppm was supposed to be a triplet since the carbon atom close to the C=O has two neighbouring protons. The triplet from 3.6 ppm-3.9 ppm represents the carbon atom on the cyclohexane ring close to the C-O bond and has two neighbouring protons. The rest of the peaks upfield are for the protons attached to the carbon atom far away from the oxygen atom. Peak at around 173ppm represents the carbon atom of the amide and that one around 168ppm is for carbon atom of the ester. The peak at 91ppm represents the atom bonded to the oxygen atom. The rest of the peaks upfield are from the alkane chain part of ergocalciferol.
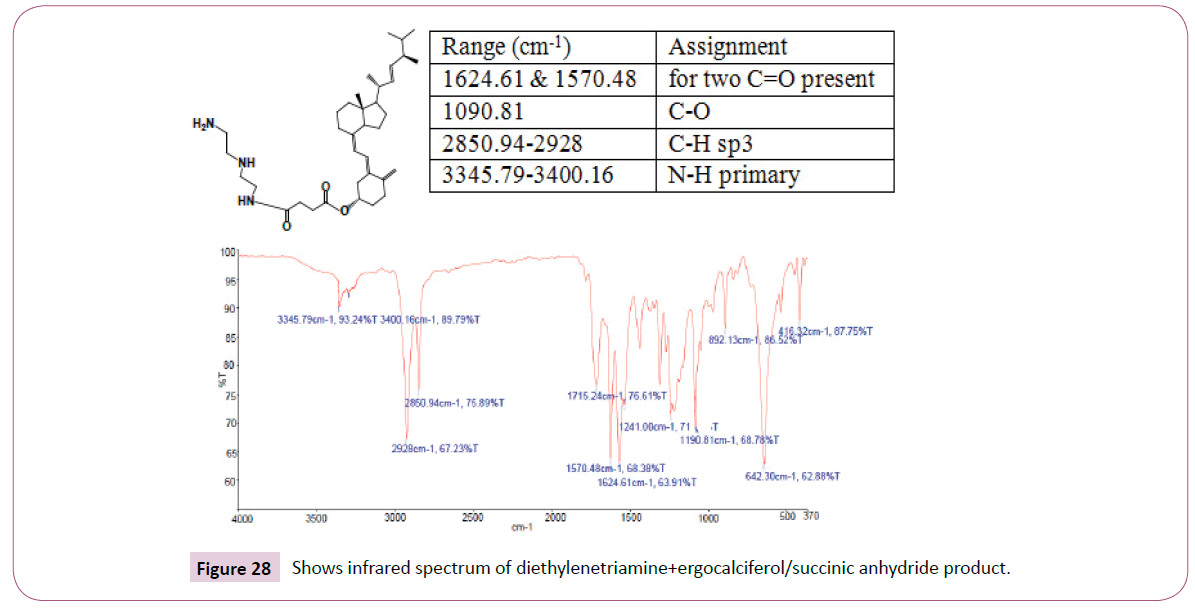
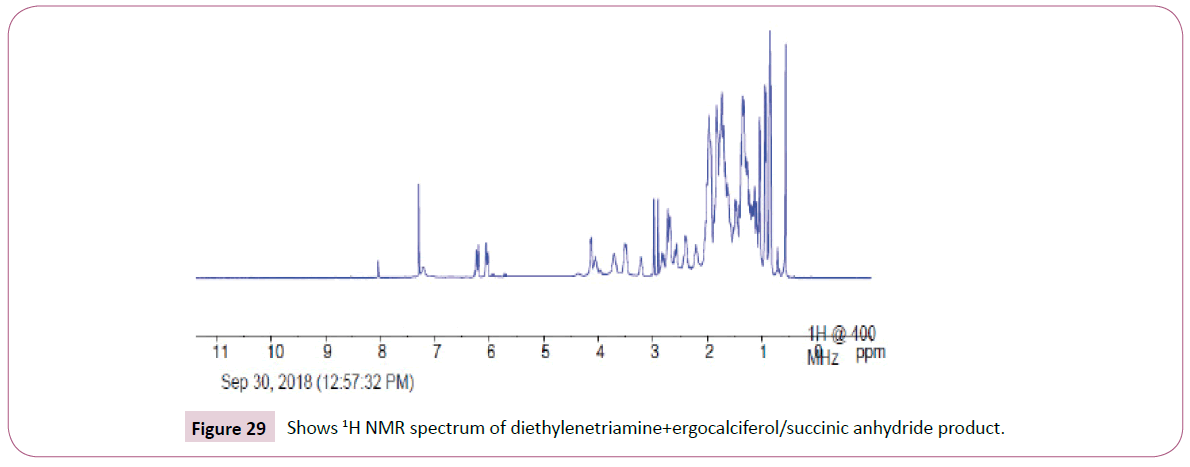
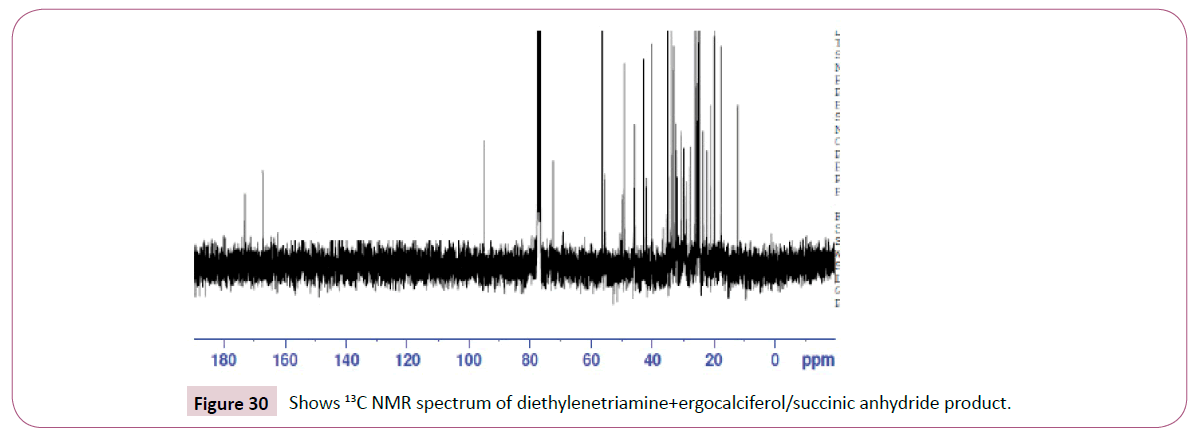
The peak at around 8.0 ppm represents the proton of the NH group and the other one at 7.2 ppm is for the NH2 part. The signal at around 6.2 ppm is for protons on the carbon atom close to the C=O bond also the signal at 6.0 ppm is for protons on the carbon atom bonded to the nitrogen atom. The peaks at 3.9 ppm represents the carbon atom on the cyclohexane ring close to the C-O bond and has two neighbouring protons. The rest of the peaks upfield are for the protons attached to the carbon atom far away from the oxygen atom. The peak at 173 ppm represents the carbon atom of the amide and that one around 162 ppm is for carbon atom of the ester. The peak at 92 ppm represents the atom bonded to the oxygen atom. Two peaks in the range 73-78 ppm represents carbon atoms; one bonded to the nitrogen atom of the NH2 and the other to the NH bond part of diethylenetriamine. The other peak at 70 ppm is for carbon atom next to the C=O, while the peak at 57 ppm is for carbon atom in the cyclohexane ring close to the C-O bond. The rest of the peaks upfield are from the alkane chain part of ergocalciferol (Figures 27 -31).
The peak at around 7.6 ppm represents the proton of the NH group. Triplet in the region of 4.0-5.0 ppm is for protons at the carbon atom next to C=O since it has two neighbouring protons.
The triplet from 3.2 ppm-3.9 ppm represents the carbon atom on the cyclohexane ring close to the C-O bond and has two neighbouring protons. The rest of the peaks upfield are for the protons attached to the carbon atom far away from the oxygen atom.
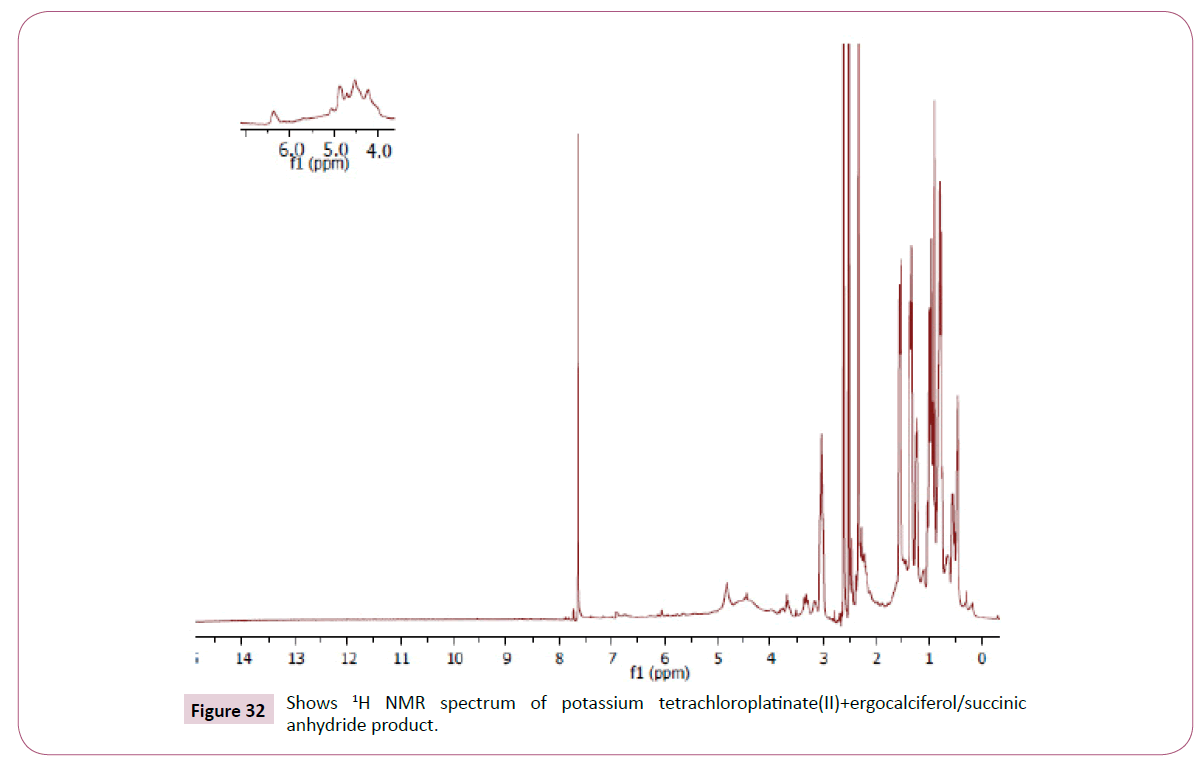
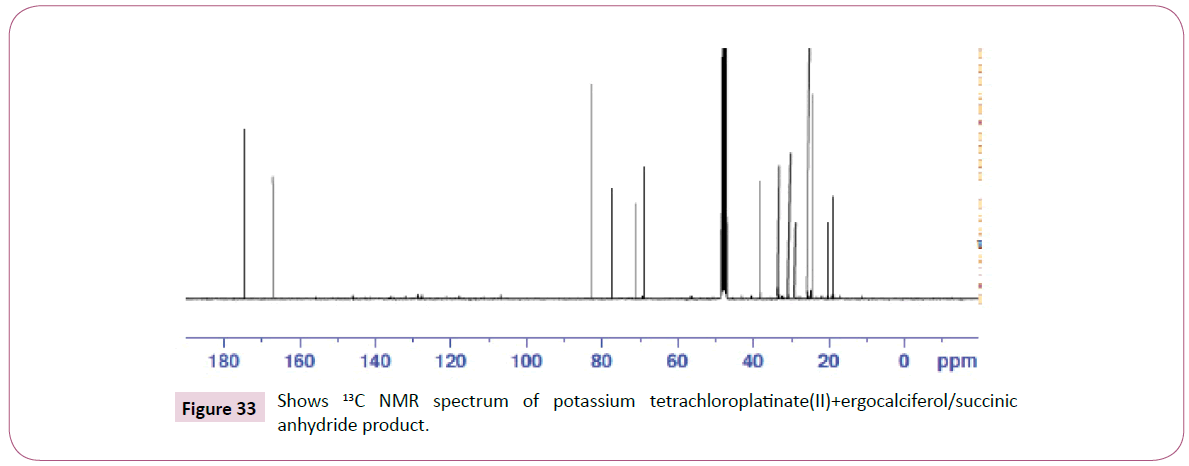
Peak at around 175 ppm represents the carbon atom of the amide and that one around 165 ppm is for carbon atom of the ester. The peak at 83 ppm represents the atom bonded to the oxygen atom. Two peaks in the range 73-78 ppm represents carbon atoms; one bonded to the nitrogen atom and the other to the NH bond part of diethylenetriamine. The other peak at 74 ppm is for carbon atom next to the C=O, while the peak at 71 ppm is for carbon atom in the cyclohexane ring close to the C-O bond. The rest of the peaks upfield are from the alkane chain part of ergocalciferol (Figures 32 and 33).
Conclusion
All hybrid compounds were synthesized at room temperature and the obtained percentage yield is in the range of 70-90%. The progress of these reactions was monitored using a thin layer chromatography and the results obtained indicates that the reactions were successfully completed due to the formation of a single spot, with an exception of compounds formed with the reaction of inorganic compounds as inorganic compounds are UV inactive. Further identification of the synthesized compounds was performed and the Infrared, 13C NMR and 1H NMR results indicates that the vitamin D hybrid compounds can be successfully synthesized from amidation and esterification reactions.
References
- Cremers SC, Pillai G, Papapoulos SE (2015) Pharmacokinetics/pharmacodynamics of bisphosphonates: use for optimisation of intermittent therapy for osteoporosis. Clin Pharmacokinet 44: 551-570.
- Ringle K (2009) An Investigation of Bone Mineral Density and Bone Mineral Content among Hispanic Women by Lifestyle Factors (Doctoral dissertation, The Ohio State University).
- D'Ambrosi R, Ragone V, Caldarini C, Serra N, Usuelli FG, et al. (2017) The impact of hereditary multiple exostoses on quality of life, satisfaction, global health status, and pain. Arch Orthop Trauma Surg 137: 209-215.
- Silverwood B (2003) Building healthy bones: Preventing osteoporosis in later life requires attention to diet and exercise in childhood (Health Promotion). Paediatric Nursing 15: 27-29.
- Kling JM, Clarke BL, Sandhu NP (2014) Osteoporosis Prevention, Screening, and Treatment: A Review. J Womens Health (Larchmt) 23: 563-572.
- Ackerman LV, Del Regato JA, Spjut HJ (2018) Cancer: diagnosis, treatment, and prognosis. 783 St. Louis: USA.
- Coleman RE (2006) Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 12: 6243s-6249s.
- Creagan ET, Pruthi S (2018) Bone cancer-Symptoms and causes - Mayo Clinic.
- Kuhlmann L, Poulsen JL, Kohler M, Rasmussen HH, Vestergaard P, et al. (2018) Osteoporosis in Chronic Pancreatitis Outpatients Associates withSeveral Risk Factors. J Pancreas 19: 183-189.
- USDHHS (2004) Bone Health and Osteoporosis: A Report of the Surgeon General. Office of the Surgeon General, Rockville, MD, USA.
- Omsland TK, Holvik K, Meyer HE, Center JR, Emaus N, et al. (2012) Hip fractures in Norway 1999-2008: time trends in total incidence and second hip fracture rates: a NOREPOS study. Eur J Epidemiol 27: 807-814.
- Sözen T, Özışık L, Başaran NC (2017) An overview and management of osteoporosis. Eur J Rheumatol 4: 46-56.
- Grey A, Reid IR (2006) Differences between the bisphosphonates for the prevention and treatment of osteoporosis. Ther Clin Risk Manag 2: 77-86.
- Ezra A, Hoffman A, Breuer E, Alferiev IS, Mönkkönen J, et al. (2000) A Peptide Prodrug Approach for Improving Bisphosphonate Oral Absorption. J Med Chem 43: 3641-3652.
- Russell RG, Croucher PI, Rogers MJ (1999) Bisphosphonates: pharmacology, mechanisms of action and clinical uses. Osteoporos Int 9: S66-80.
- Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, et al. (1999) Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest 104: 1363-1374.
- Kondrasenko I (2012) Synthesis of partial bisphosphonate esters with single side chain. University of eastern finland, Finland, Europe.
- Robinson J (2016) Vitamin D Council: How do I get the vitamin D my body needs?
- Vermaat H, Kirtschig (2008) Prevention and treatment of glucocorticoid-induced osteoporosis in daily dermatologic practice. Int J Dermatol 47: 737-742.
- Hernlund E, Svedbom A, Ivergard M, Compston J, Cooper C, et al. (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 8: 136.
- Russell RG, Rogers MJ (2001) Bisphosphonates: from the laboratory to the clinic and back again. Bone 25: 97-106.
- Naydenova E, Ancheva MT, Todorov P, Yordanova TS, Troev K (2006) Novel α-aminophosphonic acids. Design, characterization, and biological activity. Med Chem 14: 2190-2196.
- Rodan GA, Martin TJ (2000) Therapeutic approaches to bone diseases. Science 289: 1508-1514.
- Drake MT, Clarke BL, Khosla S (2017) Bisphosphonates: mechanism of action and role in clinical practice Mayo Clin Proc 83: 1032-1045.
- McAlister, Dietz ML, Chiarizia R, Herlinger AW (2001) Metal ion extraction by silyl- substituted diphosphonic acids. I. P,P’-Di-[3-(trimethylsilyl)-1-propylene] methylene- and ethylidenediphosphonic acids. Sep Sci Technol 36: 3541-3562.
- https://www.cancer.org/cancer/bonecancer/treating/chemotherapy.html
- https://www.iofbonehealth.org/facts-statistics
- Kamau E (2011) Osteoporosis in the elderly, pharmacological and non-pharmacological prevention and treatment. Arcada, Finland, Europe.
- Ntiamoah FK (2016) Use of anti-osteoporosis drugs in The Tromsø Study: Is undertreatment a problem? Norges arktiske Universitet, Norway.
- Odawa F, Ojwang S, Muia N (2004) The prevalence of postmenopausal osteo-porosis in black Kenyan women. J Obstet Gynaecol 17: 45-46.
- Ranu BC, Dutta P (2003) A simple and convenient procedure for the conversion of esters to secondary amides.
Synth Commun 33: 297-301. - Reid IR, Brown JP, Burckhardt P, Horowitz Z, Richardson P, et al. (2002) Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med 346: 653-661.
- Palacios F, Alonso C, de Los Santos JM (2005) Synthesis of beta-aminophosphonates and -phosphinates. Chem Rev 105: 899-931.
- Shriner RL, Fuson RC, Curtin DY (1956) The systematic identification of organic compounds. John Wiley & Sons, Inc, New York.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences