ISSN : 0976-8505
Der Chemica Sinica
Synthesis and Biological Evaluation of Some Novel Substituted Pyrrolizines and Pyrimidopyrrolizines as Chemotherapeutic Agents
Samir M El-Moghazy1, Mona M Hanna1, Awatef El-Said Farag1 and Amany Belal2*
1Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Cairo University, Cairo 11562, Egypt.
2Medicinal Chemistry Department, Faculty of Pharmacy, Beni-Suef University, Beni-Suef 62514, Egypt.
Abstract
A novel series of 3-ethyl ester substituted pyrrolizines (4, 5, 8-11, 14a,b, 15-19c), 3-oxadiazinyl substituted pyrolizines (21-23b, 25-27b), 3-carboxilic acid substituted pyrrolizine (7) and pyrimidopyrrolizines (6, 12, 14a,b, 24a,b, 28a,b) were synthesized. Their chemical structures were confirmed by spectral and elemental analysis. Ten compounds of these new derivatives showed activity against MCF7 cancer cell line with IC50 value less than 60 μM. Moreover, compound ethyl 2-{[aminomethylene]amino}-1-cyano -6,7-dihydro-5H-pyrrolizine-3-carboxylate (11) that was prepared by facile chemical reaction of the ethyl ester start 3 with formamide, showed activity on candida albicans equipotent with that of clotrimazole.
Keywords
Pyrrolizine, Pyrimidopyrrolizine, Oxadiazine, Ethyl ester, Antitumor, Antimicrobial, Antibacterial, Antifungal
Introduction
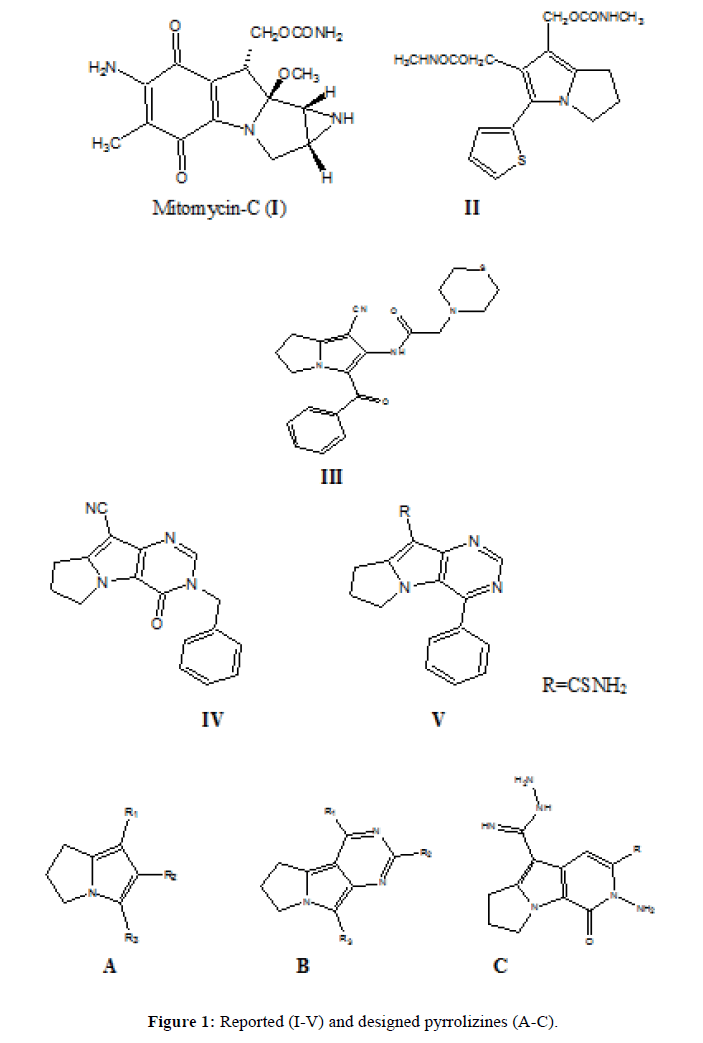
The pyrrolizine derivatives constitute an important group of heterocyclic compounds. Pyrrolizidine skeleton is a naturally occurring system; it was separated from a number of plants [1] in the form of alkaloids [2-4]. Pyrrolizine derivatives which are not naturally occurring have attracted considerable attention in medicinal chemistry as well. They have several pharmacological activities as antineoplastic activity [5-7], anti-inflammatory [8-10], antiviral [11,12], CNS activity [13-17] and immunomodulator activity [18].
Mitomycin-C, Figure 1(I), is naturally occurring and has a pyrrolizine scaffold in its structure. It is an antibiotic and anticancer drug at the same time, it is used for treatment of esophageal carcinoma, anal cancers, breast cancers and superficial bladder tumors. Mitomycin-C 0.02% can be applied topically in eye surgery in order to prevent scarring during glaucoma [19,20]. It also has strong bactericidal action against both gram-negative and gram-positive organisms [21]. 5-thienyl 2,3-dihydro-1H-pyrrolizine derivatives Scheme 1 (II) showed antitumor activity comparable with that of mitomycin [22]. Also compound N-(3-Benzoyl-1-cyano-6,7-dihydro-5H-pyrrolizin-2-yl)-2-thiomorpholin- 4-yl-acetamide Figure 1 (III) was reported to have a broad spectrum anticancer activity against breast (MCF7), colon (HCT116) and liver (HEPG2) cancer cell lines [23]. In addition, clazamycins A and B are pyrrolizidines with antitumor and antibiotic activity [24]. Moreover, Pyrimido (4,5-b) pyrrolizine derivative Figure 1 (IV) showed neoplasm inhibiting activity [25] and compound Figure 1 (V) showed significant antitumor activity in vivo [26].
Based on these findings that proved the importance of this heterocyclic scaffold, we designed and synthesized new series based on pyrrolizine as the main skeleton and with different substituents at position 1, 2 and 3, the synthesized new derivatives general formula A, B and C are represented in Figure 1. Exploration of the biological activity on MCF7 cancer cell line and also the antimicrobial activity were discussed in this study.
Materials and Methods
Chemistry
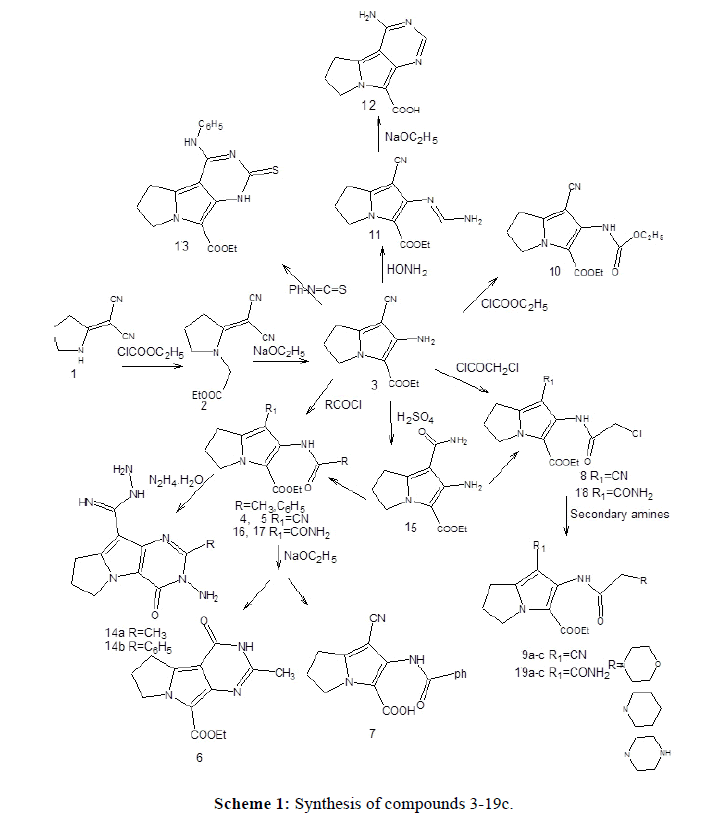
Melting points are uncorrected and were determined by open capillary tube method using Electrothermal IA9100MKdigital melting point apparatus. Elemental microanalyses were performed at the micro analytical center, Faculty of Science, Cairo University. The infrared (IR) spectra were recorded on Schimadzu-435 IR and Bruker FT-IR spectrophotometers and expressed in wave number (cm-1) using potassium bromide disc. The proton nuclear magnetic resonance (1H-NMR) spectra were recorded on a varian mercury spectrophotometer at 300 MHZ using tetramethylsilane (TMS) as internal reference. Chemical shift values were given in parts per million (ppm). Mass spectra were performed on Fennigan MAT, SSQ 7000 mass spectrophotometer at 70 eV. IUPAC chemical nomenclature were assigned using ACD/1-Labs program, version 8 (1995). Pyrrolidin-2-ylidenemalononitrile (1) was prepared according to reported procedure from 2-pyrrolidinone, its m.p. is 159-61° (reported 158-9°) [27,28]. Compound 3 also have been previously reported [25].
Ethyl [2-(dicyano methylene) pyrrolidin-1-yl] acetate (2)
A mixture of compound 1 (1 g, 7.5 mmol), powdered anhydrous potassium carbonate (2.1 g, 15 mmol) and ethylchloroacetate (0.92 g.,7.5 mmol.) in dry acetone (30 ml) was stirred under reflux for 8 hours and filtered. The filtrate was concentrated and set aside to cool; the formed white crystals were collected, dried, and recrystallized from ethanol. Yield (72%), m.p. 87-89°C. IR(cm-1): 2986 (CH2, CH3), 2211, 2190 (2CN), 1750 (CO).
Ethyl 2-amino-1-cyano-6,7-dihydro-5H-pyrrolizine-3-carboxylate (3)
Compound 2 (1g, 4.5mmol) was treated with 1% sodium ethoxide solution (0.3 g sodium metal in 30 ml absolute ethanol) at room temperature. The obtained crystals were collected, dried and washed with ethanol, yield (90%), m.p. 237-239°C (reported 239-41°C ) I R (cm-1): 3431,3338 (NH2), 2982 (CH2,CH3), 2210 (CN), 1668 (CO).
Ethyl 2- (acetylamino)-1-cyano-6,7-dihydro-5H-pyrrolizine-3-carboxylate (4)
A mixture of compound 3 (1 g, 4.5 mmol) and acetyl chloride (0.7 g, 9 mmol) in dioxan (15 ml) was stirred for 24 hours at room temperature. The formed white crystals were filtered, dried and recrystallized from acetone. Yield (83%), m.p. 137-139. I R (cm-1): 3268 (NH), 2228 (CN), 1697 (COOC2H5), 1668 (CONH). 1H-NMR (DMSO-d6): δ = 1.24 (q, 3H, CH2CH3), 2.01 (s, 3H, COCH3), 2.49 (m, 2H, C6), 2.94 (t, 2H C7), 4.19 (m, 4H,C5, CH2CH3), 9.56 (s,1H, NH). Microanalysis (%): Calculated for C13H15N3O3 (261.28) Cal. C 59.75, H 5.78 N 16.08 Found, C 59.76 H 5.94 N 15.93.
Ethyl 2-(benzoylamino)-1-cyano-6,7-dihydro-5H-pyrrolizine-3-carboxylate (5)
A mixture of compound 3 (1 g, 4.5 mmol) and benzoyl chloride (1.26g, 9 mmol) in dioxin, was refluxed for 3 hours, then cooled to room temperature. The formed crystals were filtered, dried and recrystallized from ethanol. Yield (78%), m.p. 193-195. I R (cm-1): 3295 (NH), 2221 (CN), 1662 (2CO). MS: m/z (%), M+ 323 (43); 105 (100). Microanalysis (%): Calculated for C18H17N3O3 (323.35), Calc, C 66.86 H 5.29 N 12.99, Found, C 67.01H 5.11 N 12.89.
Ethyl 3- methyl-1-oxo-2,7,8,9- tetrahydro-1H- pyrimido[5,4-a]pyrrolizine-5-carboxylate (6)
A solution of compound 4 (1 g) in 0.5% sodium ethoxide was refluxed for 2 hours, the formed crystals were filtered, dried and recrystallized from methanol. Yield (70%), m.p. 316-318°C. IR (cm-1): 3146 (NH), 2969, 2927 (CH aliphatic), 1672 (COOC2H5), 1615 (CONH). MS: m/z (%), M+ 261 (70) ; 189 (100). 1H-NMR(DMSOd6), 1.25 (t, 3H, CH2CH3), 2.25 (s, 3H, CH3), 2.49 (m, 2H, C8), 3.05 (t, 2H, C9), 4.23 (q, 2H, CH2CH3), 4.37 (t, 2H, C7),11.45(s,1H,NH). Microanalysis (%): Calculated for C13H15N3O3; ( 261.28 ), C 59.76 H 5.78 N 16.08, Found C 59.58 H 5.37 N 16.20.
2- (Benzoylamino)-1-cyano-6,7-dihydro-5H-pyrrolizine-3-carboxylic acid (7)
Compound 5 (0.5 g, 3.38 mmol) in 0.5% sodium ethoxide solution was refluxed for 2 hours, the formed product was collected and dissolved in water (30 ml), the solution was neutralized with dilute HCl. The produced precipitate was filtered, dried and recrystallized from acetone. Yield (46%), m.p. 199-201°C. IR (cm-1): 3340 (NH), 2800-3500 (OH stretching), 2238 (CN), 1662(COOH,CONH). MS: m/z (%), M+ 295 (3); 106 (100). Microanalysis (%): Calculated for C16H13N3O3. 1.5 H2O (322), C 59.62 H 5.00 N 13.03, Found C 59.43 H 5.37 N 12.55.
Ethyl 2-[chloroacetamido]-1-cyano-6,7-dihydro-5H-pyrrolizine-3-carboxylate (8)
A mixture of compound 3 (1 g, 4.5 mmol.) and chloroacetyl chloride ( 1 g, 9 mmol.) in dioxan was stirred at room temperature for 24 hours, the formed white precipitate was filtered, dried and recrystallized from acetone. Yield (89%), m.p. 176-178°C IR (cm-1) : 3240 (NH), 2982,2961 (CH aliphatic), 2225 (CN), 1701 (COOC2H5), 1683 (CONH), 769 (Cl). 1H-NMR (DMSO-d6); δ=1.25 (t,3H,CH2CH3), 2.47 (m,2H,C6), 2.98 (t,2H,C7), 4.21(m,4H,C5,CH2CH3), 4.32(s,2H,CH2Cl), 9.99(s,1H,NH). Microanalysis (%): Calc. for C13H14CLN3O3 (295.73), C 52.79 H 4.77 N 14.20, Found C 52.52 H 4.84 N 14.12.
General method for the preparation of compounds 9a-c
A mixture of compound 8 (1g, 3.4mmol) and the secondary amine (8 mmol) in absolute ethanol (20 ml) was refluxed for 8 hours. The reaction mixture was filtered while hot and left to cool; the separated crystals were collected, dried and crystallized from ethanol.
Ethyl 1-cyano-2-[morpholin-yl acetamido]-6,7- dihydro-5H- pyrrolizine-3-carboxylate (9a)
White crystals, yield (68%), m.p. 167-168. IR (cm-1): 3299(NH), 2984, 2918 (CH aliphatic), 2223(CN), 1683(2CO). MS: m/z (%), M+1 347 (2.7); 100 (100). 1H-NMR(DMSO-d6); δ=1.27(t,3H,CH2CH3), 2.49(m,2H,C6), 2.99(t,2H,C7), 3.14(s,2H,COCH2N), 3.67(2t, 8H,O(CH2)4N), 4.28(m,C5,CH2CH3), 10.14(s,1H,NH). Microanalysis(%) cal. For, C17H22N4O4(346.39), C 58.94, H 6.40 N 16.17 found, C 58.75 H 6.62 N 16.20.
Ethyl 1-cyano-2-[piperidinyl acetamido]-6,7-dihydro-5H-pyrrolizine-3-carboxylate (9b)
White crystals, yield (74%), m.p. 167-169, IR (cm-1): 3227 (NH), 2976, 2940 (CH aliphatic), 2225 (CN), 1703(COO), 1673 (NHCO). MS: = m/z (%) = M+1 347 (2.7); 100 (100). Microanalysis (%) cal. For C18H24N4O3 (344.41), calc. C 62.77 H 7.02 N 16.26, Found, C 62.30 H 7.36 N 16.15.
Ethyl 1- cyano-2-[piperazin-yl acetamido] -6,7- dihydro-5H-pyrrolizine-3-carboxylate (9c)
White crystals, yield (73%), m.p. 199-200, MS: m/z (%), M+1 347 (2.7) ; 100 (100). Microanalysis(%) cal. For C17H23N5O3 (345.40), calc. C 59.11 H 6.71 N 20.27, found C 59.12 H 6.41 N 20.18.
Ethyl 1- cyano-2- [(ethoxycarbonyl)amino]-6,7-dihydro-5H-pyrrolizine-3-carboxylate (10)
A mixture of compound 3 (1 g, 4.5 mmol) and excess ethylchloroformate (10 ml) was refluxed for 5 hours, the formed crystals were filtered, dried and recrystallized from acetone. Yield 76 (%), m.p. 143-144°C. IR(cm-1): 3318(NH), 2978, 2936 (CH aliphatic), 2223(CN), 1742 (COOC2H5), 1668 (NHCOOC2H5). Microanalysis(%): calculated for C14H17N3O4(291.31), C 57.72 H 5.88 N 14.42, Found C 57.75 H 5.74 N 14.13.
Ethyl 2-{[aminomethylene]amino}-1-cyano-6,7-dihydro-5H-pyrrolizine-3-carboxylate (11)
A mixture of compound 3 (1 g, 4.5 mmol) and formamide (80%) (30 ml) was refluxed for 20 hours, the reaction mixture was poured over water (50 ml) then cooled. The produced precipitate was filtered, dried, and recrystallized from ethanol. Yield (64%), m.p.245-247°C. I R (cm-1):3502, 3420(NH2), 2982, 2935 (CH aliphatic), 2220 (CN), 1677(CO). MS: = m/z (%) = M+ 246 (30) ; 174 (100). 1H-NMR (DMSO-d6); δ=1.26(t,3H,CH2CH3), 2.37(m,2H,C6), 2.85(t,2H,C7), 4.07(t,2H,C5), 4.19(q,2H,CH2CH3), 5.75(s,2H,NH2), 8.09(s,1H,CH-NH2) Microanalysis(%): Calculated. for C12H14N4O2 (246.27), Calc. C 58.52 H 5.72 N 22.75, Found C 58.82 H 5.44 N 22.46.
1-Imino-2,7,8,9-tetrahydro-1H-pyrimido[5,4-a]pyrrolizine-5-carboxylic acid (12)
The solution of compound 11 (1g, 4.1mmol) in 0.5% sodium ethoxide (0.15 g in 30 ml absolute ethanol) was refluxed for 3 hours, the formed crystals were collected, dissolved in water then neutralized with dil HCl, the liberated solid was filtered, dried and recrystallized from ethanol/acetone. Yield (56%), m.p. 266-268 °C I R (cm-1) : 3314 (NH2), 2800-3400 (OH stretching), 1656 (CO). Microanalysis(%): calculated for C10H10N4O2. (218.22), Calc. C 55.04 H 4.61 N 25.67, Found C 55.01 H 4.14 N 25.38.
Ethyl 1-imino-2-phenyl-3-thioxo-2,3,4,7,8,9-hexahydro-1H-pyrimido[5,4-a]pyrrolizine-5-carboxylate (13)
Compound 3 was refluxed in excess phenylisothiocyanate (10 ml) for 3 hours, the formed crystalline product was filtered, dried and recrystallized from acetone. Yield (69%), m.p. 243-245°C IR(cm-1): 3380, 3258(2NH), 3091(CH,aromatic), 2985, 2927(CH aliphatic), 1678(CO). MS: m/z (%), M+ 354 (100) ; 354 (100). 1H-NMR(DMSO-d6); δ=1.29 (t, 3H, CH2CH3), 2.48(m, 4H, C6, C7), 4.29 (m,4H,CH2CH3,C5), 7.19-7.39 (m,5H,aromatic), 7.42(s,2H,2NH). Microanalysis (%): Calculated for C18H18N4O2S.H2O (372.45), Calc. C 58.04 H 5.41 N 15.04, Found C 58.49 H 5.10 N 15.54
General method for the preparation of 14a,b
A mixture of compound 4 (0.5 g, 1.91 mmol) or 5 (0.5 g, 3.38 mmol) and hydrazine hydrate 98%, (20 ml) was refluxed for 5 hours, then filtered. The formed white crystals were dried and crystallized from methanol.
2-Amino-3-methyl-1-oxo-4,6,7,8-tetrahydro-1H-pyrimido [4,5-b]pyrrolizine-5-carbox imidohydrazide (14a)
Yield (74%), m.p. 286-288°C IR(cm-1): 3365, 3255 (2NH2), 3205 (2NH) 2966(CH aliphatic), 1686 (C=O). 1H-NMR (DMSO-d6); δ=2.43(m,2H,C6), 2.56(s,3H,CH3), 3.05(t,2H,C7), 4.21(t,2H,C5), 5.70(s,2H,HN=C-NH), 7.17(s,2H,HNNH 2), 7.69(s,2H,N-NH2). Microanalysis (%): Calculated for C11H15N7O (261.28) cal, C 50.56 H 5.78 N 37.52, found, C, 50.77 H, 5.94 N, 37.48.>
phenyl-1-oxo-4,6,7,8-tetrahydro-1H-pyrimido[4,5-b]pyrrolizine-5-carboximido hydrazide (14b)
Yield (73%), m.p. 253-255°C IR(cm-1): 3451, 3414, 3369, 3339 (2NH2), 3315, 3186 (2NH), 1653 (C=O). MS: = m/z (%) = M+1 324 (20) ; 175 (100). Microanalysis (%): Calculated for C17H17N7O (323.35) C 59.43 H 5.29 N 30.32, found, C 59.56 H 5.30 N 30.10.
Ethyl-2-amino-1-(aminocarbonyl)-5,6-dihydro-5H-pyrrolizine-3-carboxylate (15)
A mixture of 3 (10 mmol) and 90% sulfuric acid (10 ml) was stirred for few minutes and left to stand for 48 hours at room temperature. The reaction mixture was cooled, poured over cold and stirred ammonia solution, left overnight in a refrigerator. The product was filtered, washed with water and crystallized from ethanol. Yield (77%), m.p 224-226°C. I R (cm-1): 3473, 3363, 3318, 3260 (NH2, CONH2), 2982, 2929 (CH aliphatic), 1658 (2CO). 1H-NMR(DMSO-d6); δ=1.25 (t,3H,CH2CH3), 2.34 (m,2H,C6), 2.96 (t,2H,C7), 4.03 (t,2H,C5) 4.19 (q,2H,CH2CH3), 5.87 (s,2H,NH2), 6.51 (s,2H,CONH2). Microanalysis (%) : Calculated. for C11H15N3O3 (237.26), Calc. C 55.68 H 6.37 N 17.71 Found C 55.51 H 6.62 N 17.42.
Ethyl-2-(acetylamino)-1-(aminocarbonyl)-5,6-dihydro-5H-pyrrolizine-3-carboxylate (16)
A mixture of 15 (10 mmol) and acetyl chloride (0.8 ml, 11 mmol) in dioxan (10 ml),was left to stand overnight, at room temperature. The obtained crystalline product was filtered, washed with water and crystallized from ethanol. Yield (72%), m.p 199- 201°C. I R (cm-1): 3418,3185,(NH2, NH), 1712 (CO of ester),1653 (2CO of amide). MS: m/z (%), M+ 279 (7.7); 148 (100). 1H-NMR(DMSO-d6); δ=1.29 (t, 3H, CH2CH3), 2.01 (s, 3H, COCH3), 2.46 (m, 2H,C6), 2.96(t, 2H, C7), 4.17(m, 4H,CH2CH3, C5), 9.56 (s,3H,NH,NH2). Microanalysis (%): Calculated. for C13H17N3O4 (279.30), C 55.90 H 6.13 N 15.04, Found, C 56.24 H 5.84 N 15.07.
Ethyl-1-(aminocarbonyl)-2-(benzoylamino)-5,6-dihydro-5H-pyrrolizine-3-carboxylate (17)
Compound 15 (2.37 g, 10 mmol) and benzoyl chloride (1.3 ml, 11 mmol) in dioxan (20 ml) were refluxed for 2 hours, the reaction mixture was concentrated by distillation and cooled. The separated crystals were collected, dried and recrystallized from acetone. Yield (78%), m.p 237-239°C. I R (cm-1): 3144, 3044 (NH2, NH), 1738 (COO),1653 (2CO of amide). Microanalysis (%) : Calculated. for C18H19N3O4 (341.37), C 63.33 H 5.60 N 12.30, Found, C 63.40 H 5.50 N 12.39.
Ethyl 1-(aminocarbonyl)-2-[(chloroacetyl)amino]-6,7-dihydro-5H-pyrrolizine-3-carboxylate (18)
This compound was prepared by the same method used for the preparation of 8 starting from 3 (0.9g, 3.8 mmol) and chloroacetylchloride (0.9 g, 7.6 mmol). It was crystallized from acetone. Yield (76%), m.p.211-213 ºC. IR (cm-1): 3408,3302,3195(NH2,NH), 2982(CH aliphatic), 1692(COO), 1652(2CO amide). 1H-NMR(DMSO-d6); δ=1.27(t,3H,CH2CH3), 2.36(m,2H,C6), 3.03(t,2H,C7), 4.12-4.26(m,4H,CH2CH3,C5), 4.53(s,2H,CH2Cl), 9.71(s,3H,NH2,NH). Microanalysis (%): Calculated for C13H16N3O4Cl(313.74); Calc. C 49.76 H 5.14 N 13.39 Found C 49.75 H 5.42 N 13.39.
General method for the preparation of comp. 19a-c
A mixture of 18 (2.9 mmol.) and the appropriate amine (3.5 mmol.) in absolute ethanol (10 ml) was refluxed for 8 hours. The reaction mixture was filtered while hot, concentrated and cooled. The separated crystals were collected, dried, and recrystallized from ethanol.
Ethyl-1-(aminocarbonyl)-2-[(morpholin-4-ylacetyl)amino]-6,7-dihydro-5H-pyrrolizine-3-carboxylate (19a)
White crystals, Yield (83%), m.p 219-221°C. I R (cm-1): 3567, 3488, 3158 (NH2,NH), 1669, 1610 (3CO). MS: = m/z (%) = M+1 365 (20); 77 (100). 1H-NMR (DMSO-d6); δ=1.28 (t,3H,CH2CH3), 2.49 (m,2H,C6), 3.05 (t,2H,C7), 3.37 (s,2H,COCH2N), 3.57 (2t,8H,N(CH2)4O),4.20 (q,2H,CH2CH3), 4.39(t,2H,C5), 11.05(s,3H,NH2,NH). Microanalysis (%): Calculated for C17H24N4O5 (364.40), C 56.03 H 6.63 N 15.37, found, C 55.67 H 7.08 N 15.30.
Ethyl-1-(aminocarbonyl)-2-[(piperidin-4-ylacetyl)amino]-6,7-dihydro-5H-pyrrolizine-3-carboxylate (19b)
White crystals, Yield (72%), m.p. 229-231°C. IR (cm-1): 3349, 3272, 3158 (NH2, NH), 1671, 1611(3CO). Microanalysis (%): Calculated for C18H26N4O4(362.43) C 59.65 H 7.23 N 15.45, found C 59.82 H 6.94 N 15.98.
Ethyl-1-(aminocarbonyl)-2-[(piperazin-4-ylacetyl)amino]-6,7-dihydro-5H-pyrrolizine-3-carboxylate (19c)
White crystals, Yield (79%), m.p 253-255° C. I R (cm-1): 3416, 3263, 3154 (NH2,NH),1714,1686,1650 (3CO). Microanalysis (%): Calculated for C17H25N5O4 (363.42), C 56.18 H 6.93 N 19.26. Found, C 56.21 H 7.01 N 19.25
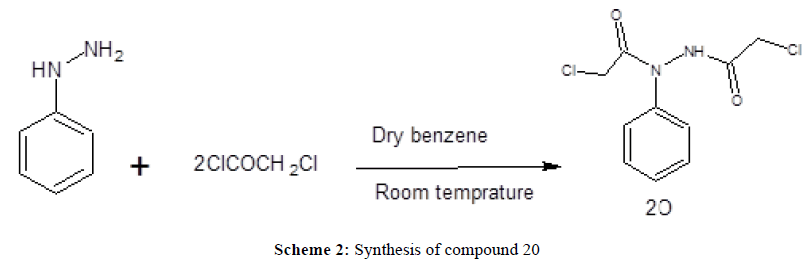
2-Chloro-N'-(chloroacetyl)-N-phenylacetohydrazide (20)
To a mixture of phenylhydrazine (1 g, 9.3 mmol) in dry benzene (20 ml), chloroacetylchloride (2.19, 19 mmol) was added drop wise, while stirring at room temperature. Shortly after the addition, a heavy precipitate was formed and stirring was continued for an additional one hour. The separated product was filtered, washed with water, dried and recrystallized from ethanol/water. Yield (92%), m.p. 116-118°C. IR(cm-1): 3212(NH), 3005 (CHaromatic),1710, 1677(2CO), 767(Cl). MS: = m/z (%) M+ 261 (64); 215 (100). 1H-NMR (DMSOd6); δ =4.16 (s,2H,CH2Cl), 4.26 (s,2H,NHCOCH2Cl), 6.93-7.46 (m, 5H, aromatic), 8.61(s,1H,NH). Microanalysis (%): Calculated for C10H10Cl2N2O2 (261.11), Calc. C 45.99 H 3.86 N 10.72, Found C 46.01 H 4.05 N 10.55.
2-Amino-3-(5-oxo-4-phenyl-5,6-dihydro-4H-1,3,4-oxadiazin-2-yl)-6,7-dihydro-5H-pyrrolizine-1-carbonitrile (21)
A mixture of 1 (1 g, 7.5 mmol), powdered anhydrous potassium carbonate (2.1g, 15mmol), and compound 20 (1.95g., 7.5mmol) in dry acetone (50 ml) was stirred under reflux for 24 hours and filtered, The filtrate was concentrated, set aside to cool, the formed reddish brown crystals were collected, dried and recrystallized from acetone. Yield (78%), m.p. 219-221°C. I R (cm-1): 3429, 3335(NH2), 3075(CH aromatic), 2208(CN), 1688 (CO). MS: = m/z (%) = M+ 321 (100) ; 321 (100). 1H-NMR (DMSO-d6); δ = 2.36(m,2H,C6),2.88(t,2H,C7),4.14(t, 2H, C5),4.87(s,2H, OCH2CO), 5.59 (s,2H,NH2) disappears with D2o, 7.28-7.66(m,5H, aromatic). Microanalysis (%): Calculated for C17H15N5O2 (321.34), Calc. C 63.54 H 4.70 N 21.79, Found C 63.62 H 4.38 N 21.77
General method for the preparation of compo. 22a-c
A mixture of compound 21 (1 g, 3.12 mmol), glacial acetic acid (0.5 ml) and p-substitutedbenzaldhyde (3.12 mmol) in absolute ethanol (20 ml) was refluxed for 10 hours, the reaction mixture was then concentrated and set aside to cool, yellow crystals were formed, collected, dried and crystallized from ethanol.
2-{[(4-Chloro phenyl)methylene]amino}-3-(5-oxo-4-phenyl-5,6-dihydro-4H-1,3,4-oxadiazin-2-yl)-6,7-dihydro- 5H-pyrrolizine-1-carbonitrile (22a)
Yellow crystals, Yield (92%), m.p. 202-204°C. I R (cm-1): 3061(CH aromatic), 2215 (CN), 1690 (CO). MS: = m/z (%) = M+ 443 (39) ; 77 (100). 1H-NMR(DMSO-d6): δ = 2.44 (m, 2H, C6), 3.04 (t, 2H, C7), 4.29(t, 2H, C5), 4.88(s,2H,OCH2CO),7.24-7.94 (m,9H,aromatic), 8.70(s,1H,N=CH). Microanalysis (%) : Calculated for C24H18ClN5O2 (443.89) C 64.93 H 4.08 N 15.77, found, C 64.68 H 4.33 N 15.68.
2-{[(4- Bromo phenyl ) methylene ] amino } -3-( 5-oxo-4-phenyl-5,6-dihydro-4H-1,3,4-oxadiazin-2-yl)-6,7- dihydro-5H-pyrrolizine-1-carbonitrile (22b)
Yellowish green crystals, Yield (85%), m.p. 199-201°C. I R (cm-1): 3069 (CH aromatic), 2209(CN),1684(CO). Microanalysis (%): Calculated for C24H18BrN5O2 (488.34), C 59.02 H 3.71 N 14.34, Found, C 58.82 H 3.68 N 14.19.
2-{[(4- Nitro phenyl ) methylene ] amino } -3-( 5-oxo-4-phenyl-5,6-dihydro-4H-1,3,4-oxadiazin-2-yl)-6,7-dihydro- 5H-pyrrolizine-1-carbonitrile (22c)
Orange crystals, Yield (88%), m.p. 226-228°C. I R (cm-1): 3069(CH aromatic), 2209(CN),1682(CO). Microanalysis (%): Calculated for C24H18N6O4 (454.44), C 63.43 H 3.99 N 18.49, Found, C 63.22 H 4.03 N 18.34.
General method for the preparation of compounds 23a,b
To 1 g of compound 21 (3.12 mmol) in dioxan (15 ml), acyl chloride ( 3.12 mmol) was added, the reaction mixture was left to stand overnight at room temperature. The separated product was filtered, washed with water, dried and crystallized from ethanol.
N-[1-Cyano-3-(5-oxo-4-phenyl-5,6-dihydro-4H-1,3,4-oxadiazin-2-yl)-6,7-dihydro-5H-pyrrolizin-2-yl]acetamide (23a)
Yellowish crystals, Yield (75%), m.p. 264-266°C. I R (cm-1): 3310 (NH), 3070(CH aromatic), 2222(CN), 1684, 1632 (2CO). MS: = m/z (%) = M+ 363 (49) ; 321 (100). 1H-NMR (DMSO-d6);=1.97 (s, 3H, CH3), 2.38 (m,2H,C6), 3.00(t,2H,C7), 4.11(t,2H,C5), 4.75(s,2H,OCH2CO), 7.23-7.65 (m,5H,aromatic),9.45(s,1H,NH). Microanalysis (%): Calculated for C19H17N5O3(363.38), C 62.80 H 4.71 N 19.27, Found, C 63.51 H 4.62 N 19.17.
N-[1-Cyano-3-(5-oxo-4-phenyl-5,6-dihydro-4H-1,3,4-oxadiazin-2-yl)-6,7-dihydro-5H-pyrrolizin-2-yl] benzamide (23b)
Yellowish white crystals, Yield (78%), m.p. 270-272°C. I R (cm-1): 3227 (NH), 3061(CHaromatic), 2220 (CN), 1691,1674 (2CO). Microanalysis (%): Calculated for C24H19N5O3 (425.45), C 67.75 H 4.50 N 16.46, Found, C 67.30 H 4.90 N 15.98.
General method for the preparation of compounds 24a,b
A solution of compound 23 a,b ( 0.36 mmol.), in sodium ethoxide 0.5% ( 20 ml) was refluxed for 2 hours. The formed precipitate was filtered and dissolved in water (20 ml) and dilute HCl was added. The obtained solid product was filtered, dried and crystallized from ethanol.
3-Methyl-1-oxo-2,7,8,9-tetrahydro-1H-pyrimido[5,4-a]pyrrolizine-5-carboxylic acid (24a)
White crystals, Yield (46%), m.p. 251-253°C. I R (cm-1): 3400 (NH), 2800-3500(OH), 1601(2CO). Microanalysis (%): Calculated for C11H11N3O3 (233.23), C 56.64 H 4.75 N 18.01, Found, C 56.84 H 4.94 N 17.92.
3-phenyl-1-oxo-2,7,8,9-tetrahydro-1H-pyrimido[5,4-a]pyrrolizine-5-carboxylic acid (24b)
White crystals, Yield (52%), m.p. 265-267°C. IR (cm-1): 3422(NH),3200-3500(OH), 1678,1623(2CO). Microanalysis (%): Calculated for C16H13N3O3 (295.30), C 65.07 H 4.43 N 14.22, Found, C 65.46 H 4.08 N 14.19.
2-Chloro-N-[1-cyano-3-(5-oxo-4-phenyl-5,6-dihydro-4H-1,3,4-oxadiazin-2-yl)-6,7-dihydro-5H-pyrrolizin-2-yl] acetamide (25)
To compound 21 (1.2 g, 3.8 mmol) in dioxan (20 ml), chloroacetyl chloride (0.9 g, 7.6 mmol) was added, The reaction mixture was left to stand for 24 hours at room temperature. The separated product was filtered, washed with water and crystallized from ethanol. Yield (68%), m.p 228-231°C. IR (cm-1):3271 (NH), 3062 (CHaromatic), 2216(CN), 1688 (CONC6H5), 1630 (CONH), 766(Cl). 1H-NMR(DMSO-d6); δ=2.42(m,2H,C6), 2.97(t,2H,C7), 3.55(s,2H,CH2Cl), 4.23(t,2H,C5), 4.84(s,2H,OCH2), 7.28-7.65(m,aromatic), 10.21(s,1H,NH). Microanalysis(%); Calculated for C19H16ClN5O3 (397.82), Calc. C 57.36 H 4.05 N 17.60, Found C 57.60 H 4.40 N 17.33
General method for the preparation of compounds 26a-c
A mixture of compound 25 (1.2 g, 2.9 mmol) and the secondary amine (3.2 mmol) in absolute ethanol (10 ml) was refluxed for 8 hours. The reaction mixture was filtered while hot, concentrated and cooled. The separated crystals were collected, dried and crystallized from ethanol.
2-(Morpholin-4-yl)-N-[1-cyano-3-(5-oxo-4-phenyl-5,6-dihydro-4H-1,3,4-oxadiazin-2-yl)-6,7-dihydro-5Hpyrrolizin- 2-yl]acetamide (26a)
Yellowish crystals, Yield (72%), m.p 195-197°C. IR(cm-1): 3345(NH),3073(CH aromatic), 2220 (CN), 1698,1642 (2CO). MS:=m/z (%)=M+ 448 (2) ;93 (100). 1H-NMR (DMSO-d6); 2.45 (m, 2H, C6), 2.96 (t,2H,C7), 3.09(s,2H,COCH2N), 3.50-3.53 (2t,8H,N(CH2)4O), 4.21 (t,2H,C5),4.87 (s,2H, OCH2CO),7.29-7.64(m,aromatic), 9.61(s,1H,NH). Microanalysis(%); Calculated for C23H24N6O4 ( 448.48), C 61.59, H 5.39, N 18.73, found, C 61.30 H 5.11 N 18.48
2-(piperidin-1-yl)-N-[1-cyano-3-(5-oxo-4-phenyl-5,6-dihydro-4H-1,3,4-oxadiazin-2-yl)-6,7-dihydro-5Hpyrrolizin- 2-yl]acetamide (26b)
Yellowish white crystals, Yield (64%), m.p 204-206°C. Microanalysis(%); Calculated for C24H26N6O3 (446.51), C 64.55 H 5.86 N 18.82, found C 64.70, H 5.70 N 18.80.
2-(Piperazin-1-yl)-N-[1-cyano-3-(5-oxo-4-phenyl-5,6-dihydro-4H-1,3,4-oxadiazin-2-yl)-6,7-dihydro-5Hpyrrolizin- 2-yl]acetamide (26c)
Yellowish white crystals, Yield (62%), m.p 222-224°C. Microanalysis(%); Calculated for C23H25N7O3 (447.50), C 61.73 H 5.63 N 21.91, found, C 61.50 H 5.60 N 21.85.
General method for the preparation of compounds 27a,b
A mixture of compound 21 (1 g, 0.32 mmol), in dioxan (30 ml), (0.35 mmol.), phenyl or p-chlorophenylisocyanate and 3-4 drops triethylamine, was refluxed for 15 hours, concentrate, leave to set aside collect the formed crystals, dry and crystallize from acetone.
1-[1-Cyano-3-(5-oxo-4-phenyl-5,6-dihydro-4H-1,3,4-oxadiazin-2-yl)-3a,4,5,6- tetrahydropentalen -2-yl] -3- phenyl urea (27a).
Yellowish crystals, Yield (62%), m.p 249-251°C. 1688, 1648(2CO). MS:=m/z (%) IR (cm-1): 3301 (NHs), 3060 (CH aromatic), 2224 (CN)=M+ 440 (54); 77 (100). 1H-NMR(DMSO-d6) 88a; δ=2.45 (m,2H,C6), 3.02(t,2H,C7), 4.24 (t,2H,C5), 4.88 (s,2H,OCH2CO), 6.95,7.66(m,aromatic protons),8.24(s,1H,NH),8.98(s,1H,NH). Microanalysis(%); Calculated for C24H20N6O3.H2O (458.48), C 62.87 H 4.83 N18.33, found, C 63.35 H 4.56 N 17.91.
1-[1-Cyano-3-(5-oxo-4-phenyl-5,6-dihydro-4H-1,3,4-oxadiazin-2-yl) -3a,4,5,6- tetrahydropentalen -2-yl] -3- p-chlorophenyl urea (27b)
Yellowish white crystals, Yield (65%), m.p 164-166°C. IR(cm-1): 3453,3341(2NH), 2223(CN),1703,1677, (2CO). Microanalysis(%); Calculated for C24H19ClN6O3. H2O (492.92), C 58.48 H 4.29 N 17.04, found, C 58.6 H 74.61 N 17.02.
General methods for the preparation of compounds 28a,b
A solution of compound 27a,b (1 g) in 0.05% sodium ethoxide (20 ml), stirred for 2 hours at room temperature, filter, dissolve in water (15 ml), neutralize with dilute HCl. The collected precipitate, was washed with water, dry and crystallize from ethanol.
1-Imino-3-oxo-2- phenyl-1,2,4,7,8,9-hexahydro-1H-pyrimido[5,4-a]pyrrolizine-5-carboxylic acid (28a)
White crystals, Yield (46%), m.p 265-267°C. IR(cm-1): 3422(NH),2900-3500(COOH), 1650(2CO). Microanalysis(%); Calculated for C16H14N4O3 (310.31), C 61.93 H 4.54 N 18.05, found, C 62.20 H 4.77 N 18.06
1-Imino-3-oxo-2-p.chlorophenyl-1,2,4,7,8,9-hexahydro-1H-pyrimido[5,4-a]pyrrolizine-5-carboxylic acid (28b)
White crystals, Yield (48%), m.p 274-276°C. IR(cm-1): 3426(2NH),3100-3500(OH),1624(2CO). Microanalysis(%); Calculated for C16H13ClN4O3 (344.76), C 55.74 H 3.79 N 16.25, found, C 55.77 H 3.91 N 16.12.
Biological screening
Potential cytotoxicity of the selected compounds on MCF7 cancer cell line was performed using the method of Skehan et al, (1990) [29]. Cells were plated in 96-multiwell plate (104cells/well) for 24 hours before treatment with the compounds to allow attachment of cell to the wall of plate. Different concentration of the compounds under test (0, 1, 2.5, 5 and 10 μg/ml) were added to the cell monolayer. Triplicate wells were prepared for each individual dose. Monolayer cells were incubated with the compounds for 48 hours at 37 ˚C and in atmosphere of 5% CO2. After 48 hours, cells were fixed, washed and stained with sulforhodamine B stain. Excess stain was washed with acetic acid and attached stain was recovered with tri EDTA buffer. Color intensity was measured by an ELISA reader. The relation between the surviving fraction and drug concentration was plotted to get the survival curve of each tumor cell line after the specified compound. Data were reviewed, compared and the biological activity was determined.
Antimicrobial screening
The antimicrobial activity for the selected newly synthesized compounds was evaluated in vitro against Staphylococcus aureus and Bacillus subtilis (as representatives of gram positive bacteria). Escherichia coli and Pseudomonas aeruginosa (as representatives of gram negative bacteria) and the fungus Candida albicans by the MIC method. The MIC is the minimal concentration at which the microbial growth was completely inhibited. For each tested compound, a stock solution of 20000 μg/ml in dimethyl formamide (DMF) was prepared and two folds serial dilution were made in the same solvent. Each dilution was added to 10 ml of nutrient agar medium to yield final test concentrations in agar medium in range of 12, 5-400 μg/ml. Controls were prepared with the same quantities of dimethylformamide but without the tested compounds. The mixtures were mixed, poured into sterile Petri dishes and allowed to harden at room temperature. The agar surface was inoculated with 10 ml of standardized suspension of test organisms (106cell/ ml) and incubated at 37°C for 24 to 48 hours. Control plates containing tetracycline and clotrimazole were seen side by side.
Results and Discussion
Chemistry
Compound (1) was prepared according to previously reported methods [27,28]. In the present work the o-aminonitrile derivative of pyrrolizine (3) (25) was synthesized via the condensation of pyrrolidin-2-ylidenemalononitrile (1) with ethyl chloroacetate, the reaction mixture was refluxed for 24 hours in dry acetone and potassium carbonate as an acid binding agent, however, the product was obtained in a low yield. Therefore, jn a trial to improve the yield, the reaction duration was decreased to reach only 8 hours, where the uncyclized ethyl [2-(dicyanomethylene)pyrrolidin-1-yl] acetate intermediate (2) was separated. Cyclization was achieved by stirring this intermediate (2) with sodium ethoxide at r.t. to afford the bicyclic pyrrolizine derivative (3) in a good yield (Scheme 1). The IR spectrum of the pyrrolidine derivative (2) revealed a carbonyl absorption band of the acetate group at 1750 cm-1 in addition to characteristic absorption bands of the geminal cyano groups at 2190 and 2211 cm-1, this confirmed the uncyclized structure. On the other hand, the IR spectrum of the o-aminonitrile pyrrolizine derivative (3) showed the disappearance of the characteristic absorption bands of the geminal cyano group and showed only one sharp band at 2210 cm-1, indicating that cyclization happened successfully. In addition, the IR spectrum revealed the sharp stretching absorption bands of the 2-amino group at 3431 and 3338 cm-1.
In this work, the O-aminonitrile (3) (Scheme 1), was acetylated by reacting it with acetyl chloride at room temperature to give the open bicyclic 2-acetylamino pyrrolizine derivative (4). Cyclization was achieved by refluxing the obtained 2-acetylamino derivative (4) with sodium ethoxide to give the tricyclic ethyl 3-methyl-1-oxo-2,7,8,9-tetrahydro-1Hpyrimido[ 5,4-a]pyrrolizine-5- carboxylate (6). The IR spectrum of (4) showed a strong and sharp band for the cyano group at 2228 cm-1, indicating that intramolecular cyclization did not occur and that the separated compound was the uncyclized 2-acetylamino derivative (4). In addition, the IR spectrum revealed stretching bands at 1697 and 1668 cm- 1, this indicated the presence of the 2 CO groups and another absorption band at 3268 cm-1 indicating the NH group. On the other hand, The IR spectrum of compound (6) showed the disappearance of the characteristic absorption band of the cyano group. The 1H-NMR of compound (4) revealed a singlet at 2.0 ppm representing the three protons of COCH3 group. In addition, the 1H-NMR spectrum of compound (6) revealed a singlet at 2.2 ppm, corresponding to the 3 protons of N=C-CH3 group, the triplet and quartet pattern of the ethyl ester group appeared at 1.2 and 4.2 ppm.
Benzoylation was performed by reacting the o-aminonitrile derivative (3) with benzoyl chloride at elevated temperature to give 2- benzoylamino pyrrolizine derivative (5), which upon treatment with sodium ethoxide afforded the free 3-carboxylic acid derivative (7). The IR spectrum of (5) revealed the characteristic absorption band of the cyano group at 2221 cm-1. However, the IR spectrum of (7) showed the appearance of a broad band at 2800-3500 cm-1 indicating the presence of OH group in addition to the cyano band at 2238 cm-1, therefore confirming hydrolysis of the ethyl carboxylate ester group to its carboxylic acid analog. The mass spectrum of (5) and (7) showed the molecular ion peak at m/z=323 and m/z=295 respectively.
Compound (3) reacted readily with chloroacetyl chloride in dioxan at room temperature to afford the 2-chloroacetylamino pyrrolizine derivative (8), the structure of (8) was confirmed by microanalytical and spectral data. The IR spectrum showed the characteristic absorption band of the cyano group at 2225 cm-1, also it revealed absorption bands at 3240 cm-1 corresponding to the NH group and 1683, 1701 cm-1 corresponding to the two CO groups. Furthermore, the 1H-NMR spectrum showed a singlet at 4.3 ppm corresponding to the two protons of the CH2Cl group, in addition to the characteristic signals of the ethyl group at 1.25 and 4.21 ppm.
Condensation of compound (8) with excess secondary amine in absolute ethanol was performed, the IR spectrum revealed the presence of the absorption band of the cyano group at 2223 cm-1. 1H-NMR spectrum of (9a) revealed two triplets at 3.64-3.67 ppm indicates morpholino protons, a singlet at 3.25 ppm indicating COCH2N protons and the ethyl group protons at 1.27 and 4.21 ppm.
Compound (3) was refluxed in excess ethylchloroformate, the 2-ethoxycarbonylamino derivative (10) was obtained. The IR spectrum of the obtained product (10) showed an absorption band of the cyano group at 2223 cm-1, in addition it revealed another absorption band at 3318 cm-1 corresponding to the NH group and two absorption bands at 1742 and 1668 cm-1 indicating the presence of the 2 carbonyl groups.
Formamide was reacted with the aminonitrile derivative (3) in the absence of solvent to give the uncyclized aminomethyleneaminopyrrolizine derivative (11), the obtained compound was cyclized to the pyrimidopyrrolizine derivative (12) by refluxing it in a solution of sodium ethoxide. The IR spectrum of compound (11) showed the characteristic absorption band of cyano group at 2220 cm-1 and the forked absorption bands corresponding to the NH2 group appeared at 3502 and 3420 cm-1. The 1H-NMR spectrum revealed the characteristic signals of the ethyl ester group appeared as a triplet and quartet at 1.2 and 4.2 ppm, in addition to a singlet signal corresponding to one proton of the N=CH-NH2 appeared at 8.0 ppm, another singlet with an integration of two protons corresponding to the amino group appeared at 5.7 ppm. On the other hand, the IR spectrum of the tricyclic structure (12) revealed the disappearance of the cyano group and the appearance of a broad band at 2700-3500 cm-1, corresponding to an OH stretching band, indicating that hydrolysis of the ester group occurred and the free carboxylic acid derivative was obtained.
Refluxing the aminonitirile derivative (3) in excess phenyl isothiocyanate gave the tricyclic pyrimido (5,4-a) pyrrolizine derivative (13). The IR spectrum of (13) showed the disappearance of the cyano band indicating that addition of phenyl isothiocyanate occured followed by intramolecular cyclization. 1H-NMR revealed the presence of ethyl ester protons as a triplet at 1.3 ppm (CH3) and a quartet at 4.2 ppm (CH2), indicating that no hydrolysis occurred to the ester group. The mass spectrum also showed a molecular ion peak at 354 confirming the tricyclic pyrimidopyrrolizine structure.
2-acylamino pyrrolizine carboxylate derivatives (4) and (5) were refluxed in excess hydrazine hydrate. Cyclization occurred as expected but an additional molecule of hydrazine hydrate was added to the nitrile group giving the 5-carboximido hydrazide pyrimido(4,5b) pyrrolizine derivatives (14a,b). The IR spectrum of (14a) showed absorption bands for the 2 NH2 and 2 NH groups at 3365-3205 cm-1, in addition to the CO absorption band at 1686 cm-1. The 1H-NMR spectrum of methyl derivative (14a) showed no signals for the ethyl carboxylate protons but it revealed the presence of a singlet signal at 2.5 ppm with an integration of three protons corresponding to the CH3 group, another singlet at 5.7 ppm for the two protons of the 2 NH groups and two singlets at 7.1 and 7.6 corresponding to the 4 protons of the two NH2 groups. The mass spectrum of (14b) showed a molecular ion peak at 324 indicating m+1.
In the present work, ethyl 2-amino-1-(aminocarbonyl)-6,7-dihydro-5H-pyrrolizine-3-carboxylate (15) was prepared by treating the aminonitrile (3) with sulfuric acid, at room temperature, then treated with ammonia solution. The IR spectrum of this compound revealed the presence of stretching absorption bands at 3473, 3363, 3318, and 3260 cm-1 indicating the presence of two amino groups, on the other hand the nitrile absorption band was not observed. Moreover, a strong absorption band of the two carbonyl groups appeared at 1658 cm-1. The 1H-NMR revealed two singlets at 5.8 and 6.5 ppm corresponding to the 4 protons of the 2 amino groups, in addition signals of the ethyl carboxylate protons appeared as triplet and quartet at 1.29 and 4.19 ppm respectively indicating that no hydrolysis occurred to the ester group.
Acetylation to the 2-amino pyrrolizine derivative (15) was achieved by reacting it with acetyl chloride in dioxan at room temperature to give the 2-acetyl aminopyrrolizine derivative (16). Benzoylation using benzoyl chloride occurred in the same way but at elevated temperature yielding 2-benzamidopyrrolizine derivative (17). The IR spectrum of (16) showed the presence of two absorption bands at 3418, 3185 cm-1 corresponding to NH2 and NH groups, the absorption bands of the CO groups were also observed at 1712 and 1653 cm-1.The 1H-NMR spectrum of (16) revealed the presence of C2H5 group as a quartet and a triplet at 1.29 and 4.26 ppm respectively, it also showed a singlet at 2 ppm corresponding to COCH3. The mass spectrum of (16) showed a molecular ion peak at 279 that confirmed the structure.
Chloroacetylation of the 2-amino-1-carboxamidepyrrolizine derivative (15) was carried out by reacting it with chloroacetyl chloride at room temperature to yield the chloroacylamino derivative (18). The IR spectrum of (18) revealed an absorption band at 3195 cm-1 corresponding to the NH group. In addition to the two absorption bands of the carboxamide amino group at 3408 and 3302 cm-1. In addition the stretching absorption bands of the 3 carbonyl groups appeared at 1698, 1652 and 1602 cm-1.
The1H-NMR spectrum of (18) showed a singlet at 4.5 ppm corresponding to the 2 protons of COCH2Cl group, in addition to signals characterizing the ethyl group at 1.2 and 4.1ppm. The chloroacylamino derivative (18) was refluxed with excess secondary amines in absolute ethanol to obtain (19a-c), the IR spectrum of (19a) revealed the appearance of the absorption bands of the carboxamide amino group at 3567 and 3488 cm-1. Also the mass spectrum showed molecular ion peak m/z=365. The 1H-NMR spectrum of (19a) revealed the presence of two triplets at 3.37-3.59 ppm corresponding to the morpholino protons, in addition to the signals of the ethyl group at 1.28 and 4.2 ppm respectively and COCH2 signal at 3.3 ppm.
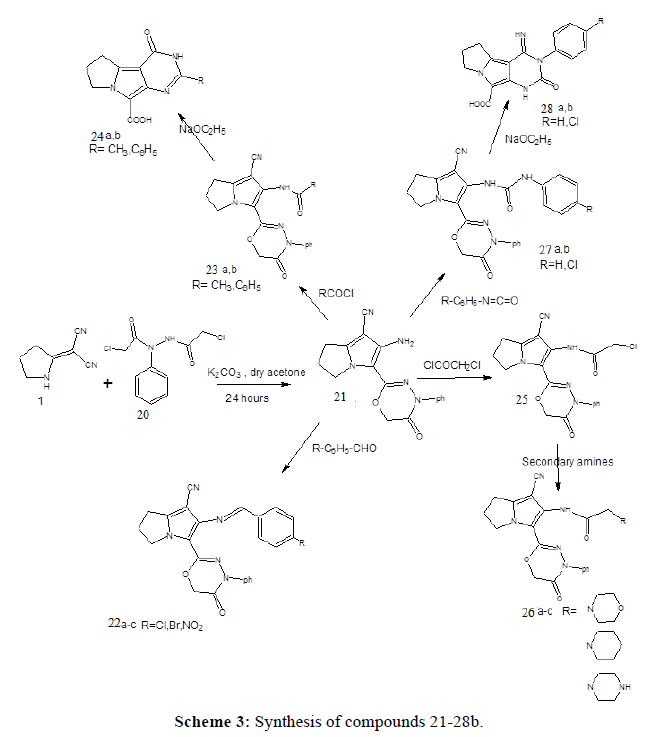
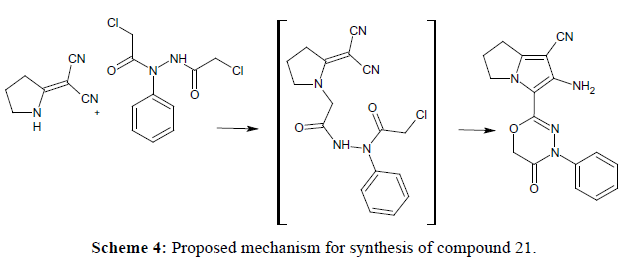
Preparation of 2-chloro-N'-(chloroacetyl)-N-phenylacetohydrazide, (20) (Scheme 2). In the present work acylation of phenylhydrazine was performed by stirring it with chloroacetyl chloride in dry benzene to yield 2-chloro-N'- (chloroacetyl)-N-phenylacetohydrazide (20). The IR spectrum of (20) showed a stretching absorption band at 3212 cm-1 corresponding to the carboxamide NH group. In addition, it revealed the absorption bands of the 2 CO groups at 1710 and 1677cm-1. The 1H-NMR spectrum revealed two singlets at 4.1 and 4.2 ppm corresponding to the 4 protons of the two CH2Cl group. Mass spectrum showed a molecular ion peak at m/z=261 confirming that chloroacetylation of phenylhydrazine occurred with 2 moles of chloroacetyl chloride. The obtained hydrazide derivative (20) was reacted with the dinitrile (1) in dry acetone and potassium carbonate as an acid binding agent. It was expected to give the 2-chloro-N'-{[2-(dicyanomethylene)pyrrolidin-1-yl]acetyl}-N-phenylacetohydrazide, however spontaneous cyclization of this intermediate occurred yielding the 3-oxadiazine pyrrolizine derivative (21). The proposed mechanism is presented in Scheme 4. The IR spectrum revealed a strong and sharp absorption band at 2208 cm-1 characteristic for the cyano group and another two absorption bands appeared at 3429 and 3335 cm-1 indicating the presence of the amino group. The 1H-NMR spectrum revealed a singlet at 4.8 ppm, that didn’t disappear upon deuteration and its integration showed two protons corresponding to the 2 protons of the OCH2CO group. On the other hand the two protons of NH2 group appeared as a singlet at 5.5 ppm that disappeared upon deuteration. The structure was also confirmed by the mass spectrum that showed the molecular ion peak at 321 m/z revealing the loss of a mole of HCl and formation of the cyclized oxadiazine derivative.
In the present work, compound (21) (Scheme 3) was condensed with para substituted benzaldehydes in absolute ethanol and few drops of glacial acetic acid, to yield the 2-(4-substituted phenyl methylene amino) derivatives (22ac). The IR spectrum of (22a) showed that the absorption band characteristic to the primary amino group at 3335 cm-1 was disappeared. The 1H-NMR spectrum showed the appearance of a singlet at 8.7 ppm, with an integration of one proton corresponding to the proton of the N=CHC6H5 group, in addition it revealed a multiplet between 7-8 ppm. Corresponding to the aromatic protons of the phenyl group.
Acetylation of the primary amino group was achieved using acetyl chloride at room temperature and the produced compound was 2-acetyl amino pyrrolizine derivative (23a). The IR spectrum of (23a) revealed the characteristic absorption band of the cyano group at 2222 cm-1, another two absorption bands were observed at 1684 and 1632 cm-1 corresponding to the 2CO groups and another peak at 3310 cm-1 indicating NH groups. The 1H-NMR spectrum of (23a) showed a singlet at 1.9 ppm, with integration of three protons indicating the protons of the methyl (COCH3) group and another singlet appeared at 9.4 ppm with integration of one proton indicating the proton of NH group, in addition to the aromatic protons appeared at 7.3-7.6 ppm and a signal for OCH2CO appeared at 4.7 ppm. Benzoylation of (21) also occurred at room temperature using benzoyl chloride in dioxan. The IR spectrum of (23b) showed absorption peak at 3227 cm-1 corresponding to NH group and the cyano group absorption band at 2220 cm-1 and also showed two absorption bands at 1691 and 1674 cm-1 corresponding to the 2CO groups. Intramolecular cyclization of the acylated compounds (23a,b), was achieved by refluxing them in sodium ethoxide solution, however the reaction was accompanied by hydrolysis of the oxadiazine ring to carboxylic acid derivatives (24a,b). The IR spectrum revealed the disappearance of the characteristic absorption band of the cyano group and the appearance of broad band at 3000- 3500cm-1 indicating the presence of OH stretching absorption band of the carboxylic group.
Acylation with chloro acetyl chloride to compound (21) occurred at room temperature in dioxan, the IR spectrum of compound (25) revealed absorption bands at 2216, 3271, 1688 cm-1 indicating CN, NH, 2CO respectively. The 1H-NMR spectrum of (25) showed a singlet at 3.58 ppm indicating two protons of CH2Cl group, in addition to the other signals characterizing the structure. The chloroacetyl chloride derivative (25) obtained was reacted with different secondary amines, the products obtained (26a-c), were also the bicyclic derivatives. The IR spectrum showed the presence of cyano group absorption band at 2220 cm-1. The 1H-NMR spectrum of morpholino derivative (26a) showed the appearance of two triplets at 3.5-3.53 ppm indicating morpholino protons. Mass spectrum of morpholino derivative showed molecular ion peak m/z=448.
In this work, addition to isocyanates in dioxan using triethyl amine produced the uncyclized urea derivatives, the IR spectrum of (27a) showed absorption bands at 2224, 3301 cm-1 indicating CN and NH group respectively and another two absorption bands at 1688 and 1648 cm-1 corresponding to 2CO groups. The 1H-NMR spectrum revealed additional aromatic protons 7-8 ppm and two singlets at 8.2 and 8.9 ppm with integration of one proton indicates NH, NH groups. Mass spectrum of phenyl urea derivative showed molecular ion peak m/z=440. Intramolecular cyclization and oxadiazine ring cleavage for compounds (27a, b) to give the free carboxylic acid group was achieved by action of sodium ethoxide to give products (28a, b), the IR spectrum of both revealed the broad stretching absorption band of the OH group and disappearance of the cyano group. Supporting data are presented in supplementary data file in the online version of the manuscript (Supplimentary Data).
Biological activity
Anticancer activity: The selected new compounds were screened on MCF7 cancer cell line using sulpharudamine-B method, the survival curve was plotted then the obtained data were expressed using death curve instead of the survival curve to provide good illustration. A plot of the concentration of the drug expressed in μg/ml against the measured test values expressed as percentage of the dead fraction was drawn after treatment of the data using a specified computerized program analysis (probit analysis) [30,31] (see Supp. Data). The obtained IC50 and IC90 values (Table 1) for the tested compounds are presented in Table 1, From the previous data It was concluded that: All the tested compounds showed good activity on MCF7 cancer cell line with IC50 values less than 60 μm. The best active compounds are 1-aminocarbonyl-3-Ethyl ester pyrrolizine substituted at position 2 with acetyl morpholine 19a and 1-cyano-3-oxadiazin pyrrolizine substituted also at position 2 with acetyl morpholine 26a with IC50 values equal 32 and 39 respectively. Both the ortho amino nitrile pyrrolizin-3-ethyl ester 3 and the benzoylated pyrrolizin-3- carboxylic acid derivative showed to be equipotent on MCF7 cancer cell line with IC50 value equal 43 μM. The other tested compounds 6, 21, 10, 9a, 14a and 15 showed a closely similar activity, their IC50 range was from 45 to 58 μM.
| Tested comp. | IC50 ( µM) | IC90 ( µM) |
|---|---|---|
| 3 | 43.195 | 93.806 |
| 4 | 55.305 | 123.316 |
| 6 | 45.047 | 94.726 |
| 7 | 43.831 | 104.068 |
| 9a | 51.589 | 91.458 |
| 10 | 49.123 | 95.843 |
| 14a | 57.142 | 101.806 |
| 15 | 58.164 | 111.102 |
| 19a | 39.215 | 72.558 |
| 21 | 46.119 | 93.608 |
| 26a | 32.042 | 62.834 |
Table 1: IC50 and IC90 of the tested compounds on MCF7 cell line
Analysis of data
Data were collected, checked, revised and analyzed by SPSS statistical package version 11. Excel computer program was used to tabulate the results and represent them graphically. Probit regression analysis procedure was introduced to select the best model that can describe the relationship between the drug concentration and the probit (percentage of protection) as a dependent variable in order to be used for prediction of the (IC50) or (IC10) of cancer cells.The in vitro growth inhibition properties of each drug were described by IC50 or IC10 the relation between drug concentration and the degree of inhibition of cancer cell line was described by the equation:
The probit (p)= intercept+regression coefficient(conc.)
Antimicrobial activity
The antimicrobial activity for the newly synthesized compounds was evaluated in vitro against Staphylococcus aureus and Bacillus subtilis (as representatives of gram positive bacteria). Escherichia coli and Pseudomonas aeruginosa (as representatives of gram negative bacteria) and the fungus Candida albicans by the MIC method. The MIC is the minimal concentration at which the microbial growth was completely inhibited. From these results (Table 2), some observations are noticed, Compounds 5, 8,10, 23a, 27a, 15, 19b inhibited the growth of Escherichia coli at 400 μg/ml, Compound 4 showed a greater activity and compound 18 was the best active one against Escherichia coli. Compounds 8, 22a, 27a,19b are active against Bacillus subtilis at 400 μg/ml and compound 14a showed better activity as it inhibited Bacillus subtilis growth at 200 μg/ml. Compounds 7, 8, 27a,18,19b are the active compounds against Staphylococcus aureus. Compounds 14a, 11, 22b, 23a, 19a are active against Pseudomonas aeruginosa and compound 19b showed to be the best active one. Compounds 7, 9b, 21, 23a, 23b, 27b, 19a are active against Candida albicans, compounds 8, 22a, 22b showed good activity, compounds 10,14a, 26a, 19b showed better activity, however, compound 11 is highly active and is found to be equipotent to the known antifungal clotrimazole. It is noticed that the antifungal activity of the tested compounds is much better than their antibacterial activity.
| Candida albicans | Escherichia coli | Pseudomonas aeruginosa | Staphylococcus aureus | Bacillus subtilis | Tested Compounds |
|---|---|---|---|---|---|
| 400 < | 200 | 400 < | 400 < | 400 < | 4 |
| 400 < | 400 | 400 < | 400 < | 400 < | 5 |
| 400 | 400 < | 400 < | 400 | 400 < | 7 |
| 200 | 400 | 400 < | 400 | 400 | 8 |
| 400 | 400 < | 400 < | 400 < | 400 < | 9b |
| 100 | 400 | 400 < | 400 < | 400 < | 10 |
| 12.5 | 400 < | 400 | 400 < | 400 < | 11 |
| 100 | 400 < | 400 | 400 < | 200 | 14a |
| 400 < | 400 < | 400 < | 400 < | 400 < | 14b |
| 400 < | 400 | 400 < | 400 < | 400 < | 15 |
| 400 < | 400 < | 400 < | 400 < | 400 < | 16 |
| 400 < | 50 | 400 < | 400 | 400 < | 18 |
| 400 | 400 < | 400 | 400 < | 400 < | 19a |
| 100 | 400 | 200 | 400 | 400 | 19b |
| 400 | 400 < | 400 < | 400 < | 400 < | 21 |
| 200 | 400 < | 400 < | 400 < | 400 | 22a |
| 200 | 400 < | 400 | 400 < | 400 < | 22b |
| 400 | 400 | 400 | 400 < | 400 < | 23a |
| 400 | 400 < | 400 < | 400 < | 400 < | 23b |
| 100 | 400 < | 400 < | 400 < | 400 < | 26a |
| 400 < | 400 | 400 < | 400 | 400 | 27a |
| 400 | 400 < | 400 < | 400 < | 400 < | 27b |
| 400 < | 12.5 | 12.5 | 12.5 | 12.5 | Tetracycline |
| 12.5 | 400 < | 400 < | 400 < | 400 < | Clotrimazole |
Table 2: Antibicrobial activity results for the tested compounds (MIC method, concentrations by μg/ml)
Conclusion
In this research work several new pyrrolizines were synthesized using accessible chemicals and facile chemical reactions, four different classes of pyrrolizines were designed and synthesized, 3-ethyl ester bicyclic substituted pyrrolizine, 3- carboxylic acid substituted pyrrolizine, 3-oxadiazinyl substituted bicyclic pyrrolizines and pyrimidopyrrolizines. Combining morpholine with 3-ethyl ester pyrrolizine 19a or 3-oxadiazinyl pyrrolizine 26a in one molecule lead to obtaining new active compounds on MCF7 cancer cell line with IC50 values equal 39 and 32 μM respectively. Moreover, compound 11 showed equipotent activity with clotrimazole against Candida albicans and this represent a good achievement for this work to synthesize a new pyrrolizine derivative with equipotent activity as clotrimazole on Candida albicans. Hoping for further investigations for this new compound 11 to be approved as an effective antifungal agent in the future.
Acknowledgement
Authors are greatful for Dr. Amal Eisa, microbiology department, faculty of Pharmacy, Beni-Suef university, Egypt, for her help in performing the antimicrobial screening of the new compounds.
References
- Singh B, Sahu PM, Singh S (2002) Antimicrobial activity of pyrrolizidine alkaloids from Heliotropium subulatum. Fitoterpia 73: 153-155.
- Smith LW, Culvenor CCJ (1981) Plant sources of hepatotoxic pyrrolizidine alkaloids. J Nat Prod 44: 129-152.
- Peter FP, Qingsu X, Lin G, Chou MW (2002) Genotoxic pyrrolizidine alkaloids - mechanisms leading to dna adduct formation and tumorigenicity. Int J Mol Sci 3: 948-964.
- Edgar JA, Roeder E, Molyneux RJ (2002) Honey from plants containing pyrrolizidine alkaloids: A potential threat to health. Agric Food Chem 50: 2719-2730.
- Wassel G, El-Menshawi B, Saeed A, Mahran G, El-Merzabani M (1987) Screening of selected plants for pyrrolizidine alkaloids and antitumor activity. Pharmazie 42: 709.
- Atwell GJ, Fan JY, Tan K, Denny WA (1998) DNA-Directed alkylating agents. 7. Synthesis, DNA interaction, and antitumor activity of bis(hydroxymethyl)- and bis(carbamate)-substituted pyrrolizines and imidazoles. J med Chem 41: 4744-4754.
- Belal A, El-Dien BM, El-Gendy (2014) Pyrrolizines: Promising scaffolds for anticancer drugs. Bioorg and Med Chemistry 22: 46-53.
- Tries S, Laufer S, Ziwon RP, Breddin HK (2002) Antithrombotic and platelet function inhibiting effects of ML3000, a new antiinflammatory drug with COX/5-LOX inhibitory activity. Inflammation Research 51: 129-134.
- Jovanovic DV, Fernandes JC, Pelletier MJ, Jolicoeur F, Reboul P, et al. (2001) In vivo dual inhibition of cyclooxygenase and lipoxygenase by ML-3000 reduces the progression of experimental osteoarthritis: Suppression of collagenase 1 and interleukin-1β synthesis. Arthritis Rheum 44: 2320-2330.
- Singh VP, Patil CS, Kulkarni SK (2006) Anti-inflammatory effect of licofelone against various inflammatory challenges. Fundam Clin Pharmacol 20: 65-71.
- Ivanenkov YA, Aladinskiy VA, Bushkov NA, Ayginin AA, Majouga AG, et al. (2017) Small-molecule inhibitors of hepatitis C virus (HCV) non-structural protein 5A (NS5A): a patent review (2010-2015). Expert Opin Ther Pat 10: 1-14.
- Oka M, Ishiwata Y, Iwata N, Honda N, Kakigami T (2001) Synthesis and anti-influenza virus activity of tricyclic compounds with a unique amine moiety. Chem Pharm Bull 49: 379-383.
- Algate DR, Augustin J, Atterson PR, Beard DJ, Jobling CM, et al. (1995) General pharmacology of [2,2-dimethyl-6-(4-chlorophenyl)-7-phenyl-2,3-dihydro-1H-pyrrolizine-5-yl]-acetic acid in experimental animals. Arzneimittelforschung 45: 159-165.
- Litvak KM, McEvoy GK (1990) Ketorolac, an injectable nonnarcotic analgesic. Clin Pharm 9: 921-935.
- Becker DP, Flynn DL, Villamil CI (2004) Bridgehead-methyl analog of SC-53116 as a 5-HT4 agonist. Bioorg & Med chem let 14: 3073-3075.
- Craig DA, Clarke DE (1990) Pharmacological characterization of a neuronal receptor for 5-hydroxytryptamine in guinea pig ileum with properties similar to the 5-hydroxytryptamine receptor. J pharmacol Exp Ther 252: 1378.
- Daveu C, Bureau R, Baglin I, Prunier H, Lancelot JC, et al. (1999) Definition of a pharmacophore for partial agonists of serotonin 5-ht3 receptors. J chem Inf Comput Sci 39: 362-369.
- Jakkampudi A, Jangala R, Reddy BR, Mitnala S, Reddy ND, et al. (2016) NF-κB in acute pancreatitis: Mechanisms and therapeutic potential. Pancreatology 16: 477-488.
- Kanzawa F, Matsushima Y, Hashi A, Shimizu E, Saijo N, et al. (1985) Evaluation of a new drug 7-N-(p-hydroxyphenyl)-mitomycin C [KW 2083] against carcinoma of the lung by the human tumor clonogenic assay. Invest New Drugs 3: 341.
- Edstrom E, YU T (1995) Ionization of Pyrido[3,4-b]pyrrolizidine and Pyrrolo[1,2-a]indole Triflate derivatives. A novel approach to the mitosene skeleton. J Org chem 60: 5382.
- Tomasz M, Lipman R, Chowdary D, Pawlak J, Verdine GL, et al. (1987) Isolation and structure of a covalent cross-link adduct between mitomycin C and DNA. Science 235: 1204-1208.
- Laduree D, Lancelot JC, Robba M, Chenu E, Math G (1989) Synthesis and evaluation of antileukemic activity of 5-thienyl- or 5-(2-furyl)-2,3-dihydro-6,7-bis(hydroxymethyl)-1H-pyrrolizine bis(alkylcarbamates) and derivatives. J Med Chem 32: 456.
- Belal A (2015) Synthesis, molecular docking and antitumor activity of novel pyrrolizines with potential as EGFR-TK inhibitors. Bioorg Chem 59: 124-129.
- Buechter DD, Hurston DE (1987) Studies on the pyrrolizidine antitumor agent, clazamycin: interconversion of clazamycins A and B. J Nat Prod 50: 360.
- Kadushkin AV, Nesterova IN, Olovko TV, Nikolaeva IS, Pushkina TV, et al. (1990) Synthesis and biologicalactivity of condensed pyrrolo[3,2-d]pyrimidines. Pharm Chem 24: 875-881.
- Mezentseva MV, Kadushkin AV, Alekseeva LM, Sokolova AS, Granik VG (1991) Synthesis and antitumor activity of pyrrolo[3,2-d]pyrimidine derivatives. Khim Farm Zh 25: 19-23.
- Etienne A, Corria Y (1969) Derivatives of 2-pyrrolidone. Bull Soc Chim 10: 3740-3712.
- Scblack P, Ricker J (1971) International journal of research in physical chemistry and chemical physics. Agew Makromolekular. Chemie 15: 203.
- Shehan P, Storeng R, Scudiero D, Monks A, Mcmahon J, et al. (1990) New colorimetric cytotoxicity assay for anticancer-drug screening. J Nat Cancer Inst 82: 1107.
- Finney DJ (1978) Statistical methods in biological assay, 3rd Ed, Griffin, London.
- Finney DJ (1979) The computation of results from radioimmunoassays. Meth Inf Med 18: 164.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences