ISSN : 2249 - 7412
Asian Journal of Plant Science & Research
Studies on the Arbuscular Mycorrhizal Biodiversity in the Plant Species of River Bhavani and its Embankments, Mettuppalyam Taluk, Coimbatore district, Tamilnadu
Shyam Praveen R*, M Santhosh Kumar, N Nagarajan and Muthuraj Kaliyappan
Department of Botany, Kongunadu Arts and Science College, Coimbatore, Tamil Nadu, India
- *Corresponding Author:
- Shyam Praveen R
Department of Botany,
Kongunadu Arts and Science College,
Coimbatore,
Tamil Nadu,
India,
Tel: 8098650633;
E-mail: shyampraveencnr@gmail.com
Received date: June 11, 2023, Manuscript No. AJPSKY-23-16958; Editor assigned date: June 14, 2023, PreQC No. AJPSKY-23-16958 (PQ); Reviewed date: June 30, 2023, QC No. AJPSKY-23-16958; Revised date: September 14, 2023, Manuscript No. AJPSKY-23-16958 (R); Published date: September 22, 2023, DOI: 10.36648/2249-7412.13.9.312
Citation: Praveen SR, Kumar MS, Nagarajan N, Kaliyappan M (2023) Studies on the Arbuscular Mycorrhizal Biodiversity in the Plant Species of River Bhavani and its Embankments, Mettuppalyam Taluk, Coimbatore district, Tamilnadu. Asian J Plant Sci Res Vol:13 No.9:312
Abstract
Arbuscular Mycorrhizal Fungi (AMF) are obligate symbionts that are predominant in the roots and soils of agricultural crop plants. AM fungi are instrumental in the survival and fitness of many plant taxa in diverse ecosystems, including many crop species. The AM symbiosis is found in roughly 70% of all plant species and is the major type of mycorrhizal symbiosis found in most tropical forests, in which there is also the greatest diversity of mycoheterotrophs. A key, universally accepted concept is that natural microbial populations in soil or other ‘living’ substrate are activated to grow around developing plant roots, giving rise to the so-called rhizosphere. As a rhizosphere develops at the root– soil interface, microorganisms there interact with both plant roots and soil constituents. This study aims to investigate to isolate and identify the Arbuscular mycorrhizal fungi from the rhizosphere soil samples of plant species in the riverbank of Bhavani, Mettupalayam Taluk, Coimbatore district and determination of the AM fungal infection in the roots of the plant species. According to this study, Thespesia populnea had the highest rate of AM fungal infection (58%) and Ipomea pestigridis had the lowest rate (16%). Colachasia sp. had the lowest spore population (121/100 g of soil), while Cassa tora had the highest (578/100 g of soil).
Keywords
Arbuscular mycorrhizal fungi, Symbiosis, Fungal infection, Glomus sps.
Introduction
Arbuscular mycorrhizal fungi have played a key role in plant evolution on earth as well as on the development and maintenance of the structure and diversity of terrestrial eco-systems [1]. Most of the plants depend on mycorrhizas to thrive particularly in fragile and stressed environment.
AM fungi colonize the root cortex, developing intercellular hyphae and extensively branched intracellular hyphae called arbuscules [2]. AM fungi promote plant growth by improved uptake of nutrients with particular emphasis on phosphorous nutrients with particular emphasis on phosphorous nutrition [3].
Under natural conditions, plant strictly speaking do not have roots, they have mycorrhizas; the roots of most flowering plants from mutuality symbioses with certain soil fungi mycorrhizal associations are found in really all ecological situations, with Arbuscular Mycorrhizae (AM) being the most common type in normal cropping systems and in natural ecosystems. AM symbiosis play a key role in nutrient cycling in ecosystems and the external mycorrhizal mycelium, in association with other soil organisms, forms water stable aggregates necessary for good quality. Arbuscular mycorrhizal fungi are ecologically important component in soil communities and AMF extensive hyphal networks could explore more soil volume where roots could not arrive [4]. AMF could transport phosphorus, nitrogen, sulphurous, water and microelements [5]. AMF may play an essential ecological role in highly stressed environments. The symbiotic association of plant and arbuscular mycorrhizal fungi association which is formed between plants and fungi has the widest distribution in nature. Mycorrhizae is considered a fundamental part of the plant as 95% of all plant species could not survive without it. India is known for its wide variety of ecological zones and natural ecosystems. To understand the ecology and function of plant fungus associations in natural ecosystems, it is necessary to clarify the seasonal diversity of AM fungi, providing insight into the factors and processes regulating ecosystem development (Figure 1).
Materials and Methods
Study area and its topography
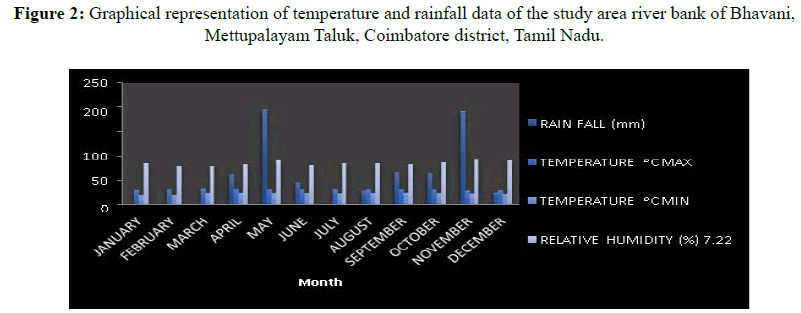
The study area is selected that the riverbank of Bhavani located near Sirumugai, Mettupalayam Taluk, Coimbatore district (Figures 2 and 3). It comprises two types of vegetation such as riparian vegetation and dry vegetation. There are two types of soils present in the hills they are clay sandy soil in the riparian vegetation and red soil in dry vegetation. It receives an annual rainfall of 187 mm. The maximum temperature occurred in the month of March 34.5°C and lowest in 19.5°C (Table 1) in the month of January, The Nilgiri hills are parted by the Bhavani river.
| Month | Rain fall (mm) | Temperature ᵒC | Relative humidity (%) 7.22 hours | |
|---|---|---|---|---|
| MAX | MIN | |||
| January | 0 | 30.1 | 19.5 | 86 |
| February | 0 | 32.2 | 20 | 80 |
| March | 3.7 | 34.5 | 23.1 | 80 |
| April | 62.7 | 32.7 | 24 | 83 |
| May | 195.8 | 32.3 | 23.5 | 91 |
| June | 46.9 | 32.3 | 23.7 | 82 |
| July | 5.1 | 32.2 | 22.9 | 85 |
| August | 28.1 | 32.3 | 23.2 | 86 |
| September | 66.2 | 33 | 23.8 | 83 |
| October | 65.2 | 31.6 | 23.3 | 87 |
| November | 191.3 | 28.6 | 22 | 93 |
Table 1: Temperature and rainfall data of the study area riverbank of Bhavani, Mettupalayam Taluk, Coimbatore district, Tamil Nadu.
Soil sample collection
Totally 25 plant species belonging to the 18 families were collected from river bank of Bhavani in the period of 2015. Root samples and rhizosphere soil samples of plant species growing in and around areas of the Bhavani river and its embankments were collected. The root and soil samples were transported to the laboratory immediately after collection.
Estimation of Arbuscular mycorrhizal colonization in roots
Root samples: Root samples, 5-15 cm long, were collected from the plant species during 2015 to 2016. During collection, care was taken to ascertain individual plants for which roots could positively identified as belonging to a particular plant species. For identification and nomenclature of the plant species the following manual was used [6].
Soil samples: The rhizospheres soil, dug up to a depth of 10 cm, was collected from each plant species after removing the surface of the soil and litter covering. These samples were kept in sterilized bags and were transported to the laboratory immediately after collection for the examination of arbuscular mycorrhizal fungal spore isolation.
Sample preservation: In the laboratory, the roots were separated from the soil, by wet sieving. The roots were washed with water and processed a fresh whenever possible. Otherwise the washed roots were fixed in Formaldehyde Acetic Acid ethanol (FAA) solution (90:5:5 V/N) as mentioned in Cade-Menun, et al. [7]. The soil sample was air dried and stored at 4°C until processed.
Each soil samples was used for chemical analysis, spore counts and classification in to various types and multiplication, concentration and separation of AM fungal spore for identification.
Preparation of soil samples for analysis: Each soil sample was spread on a flat wooden or plastic tray and was allowed to dry in air under shade. Stones and pieces of macro organic matter were removed. Large lumps were broken by hand and the soil was ground by rolling gently with a wooden roller. After grinding, the soil was screened through a 2 mm sieve and the fine soil was used for further analysis.
Soil pH: The pH of soil samples was determined (soil-water suspensions 1:5) with the help of pH meter (Elico).
Evaluation of AM infection
The root samples were cleared and stained in tryphan blue with a modified version of the Phillips and Hayman’s, et al. method. Roots were cut in to 1-2 pieces, heated at 90°C in 10% KOH for about 1 hour. For thicker and older roots, the duration was increased. The root segments were rinsed in water and acidified with dilute HCL. The root pieces were stained 0.05% tryphan blue in lacto phenol for 5 minutes and the excess stain was removed with clear lacto phenol.
The pigmented roots were heated at 90°C in 10% KOH for 2 hours, washed with fresh 10% KOH and immersed in an alkaline solution of H2O2 for 30 minutes at 25°C until bleached. They were rinsed thoroughly with water to remove the H2O2, acidified in dilute HCL and stained as described earlier. In some cases the modified method of Merry weather and Fitter, et al. was followed where autoclaving and bleaching with H2O2, were omitted. In a few cases, direct observation of unstained, fresh and intact roots was made [8].
Arbuscular mycorrhizal infection in the roots was assessed following the grid line-intersect method of Giovannetti and Mosse, et al. [9]. The stained root pieces were spread out evenly on a square plastic Petridish (10.2 × 10 cm). A grid of lines was marked on the bottom of the dish to form 1 cm inch squares. Vertical and horizontal gridlines were scanned under a dissecting microscope and the presence of infection was recorded at each point where the roots intersected a line. Four sets of observation were made, recording 100, 200, 300 and all the root gridline intersects. Each of the three replicates records was made on a fresh re-arrangement of the same root sample.
The percentage of AM infection was calculated using the formula:
Percentage of infection=(No. of root segments infected/Total no. of root segments observed) ×100
When sufficient root pieces are not available, the slide method Giovannetti and Mosse, et al. was followed. Root pieces, 1 cm long, were selected at random from a stained sample and mounted on microscope slide groups of 10. Presence of infection was recorded in each of the 10 pieces and present infection was calculated. To observe hyphae, vesicles and arbuscles under light microscope, the root pieces were mounted on glass slides either temporarily in lacto phenol. The cover slip was pressed gently to make the roots flattened and sealed with DPX medium [10].
Isolation of Arbuscular mycorrhizal spores from the soil samples
Spores were recovered from the soil samples by the wet sieving and decanting method [11]. From each soil sample, 100 g of soil was taken and mixed with 1:1 of luke warm water in a large beaker until all the aggregates dispersed to leave a uniform suspension. Heavier particles were allowed to settle down. To remove organic matter and roots, the suspension was decanted through a 710 μm sieve. The suspension that passed through 710 μm was decanted 425 μm, 250 μm, 150 μm, 75 μm and 45 μm sieves consecutively. The residues in the respective sieve were collected in petri dishes with about 10-20 ml water observed under a dissecting microscope for AM fungal spores.
The total spore count was calculated by counting the spores. Then the spores were separated using a glass pipette and segregated. The spore were mounted on clear glass slides using lacto phenol or Polyvinyl Alcohol Lacto phenol (PVL), covered with cover slips and sealed with DPX medium.
Identification of AM fungi
Based upon microscopic characters, the AM fungal spores were identified. For identification and nomenclature, keys of the following manual authors were used: Raman and Mohankumar, et al.; Schenk and Perez, et al. [12]. Classification on based on color, size, shape, surface, structure, general nature of the spore contents and hyphal attachment. Photomicrographs were taken with the help of a Magnus Olympus microscope.
Results and Discussion
The present investigation was observed the arbuscular mycorrhizal fungal infection and spore population of 25 plant species belonging to 18 families present in Table 2 and the pH also recorded in the rhizosphere soil samples (Table2). The pH ranged between 4.5 to 8.4.
Totally 25 plant species belonging to 18 families were thoroughly examined for AM fungal association. Of these, the maximum spore population was noticed in the plant species of Cassia tora (578/100 g of soil) belongs to the family Caesalpinaceae and the minimum was observed in the plant species Colachasia sp. (121/100 g of soil) belongs to
Aeraceae member. The highest AM fungal infection was recorded in Thespesia populnea (58%) belongs to
| S.no | Botanical name | Family | Soil pH | Type of infection | % of root colonization | AMF spore population/100 g of soil | AMF spore species | ||
|---|---|---|---|---|---|---|---|---|---|
| H | V | A | |||||||
| 1 | Adathoda vasica, Nees. | Acanthaceae | 4.9 | + | + | - | 23 | 261 | LABS,LDST |
| 2 | Ageratum conyzoides L. | Asteraceae | 5.2 | + | + | - | 31 | 273 | LDMR,LFSC,AAPD |
| 3 | Boerhavia diffusa L. | Nyctaginaceae | 6.7 | + | - | - | 42 | 472 | LABS,LAHTS,LAST |
| 4 | Cassia tora L. | Caesalpinaceae | 7.2 | + | - | - | 53 | 578 | LDST,LFSC,CNGR |
| 5 | Cissus quadrangularis L. | Vitaceae | 5.3 | + | - | - | 33 | 213 | LCTC,LAGR,AAPD |
| 6 | Colachia Sp. | Aeraceae | 4.9 | + | + | - | 21 | 121 | LABS,LDMR,CGRG |
| 7 | Crotalaria pallida Aiton. | Papiloniaceae | 6.1 | + | + | + | 24 | 241 | LDMK,LFSC,CNGR |
| 8 | Eragrostis uniloides Nees. | Poaceae | 7.3 | + | + | + | 26 | 321 | LCTC,LABD,LFSC |
| 9 | Ipomea pestigridis | Euphorbiaceae | 8.1 | + | - | + | 16 | 235 | LHTS,LFSC,CGRG |
| 10 | Ludwigia parvifolia Roxb. | Onagaraceae | 7.4 | + | + | - | 19 | 182 | LABS,ASCB,GRSA |
| 11 | Justicia betonica L. | Acanthaceae | 8.4 | + | + | - | 22 | 265 | LDST,LDMK,LAST |
| 12 | Ipomea cordata L. | Convolvulaceae | 5.7 | + | - | + | 17 | 164 | LAGR,LFSC,AAPD |
| 13 | Portulaca tuberosa, Roxb. | Portulaceae | 6.2 | + | + | + | 21 | 129 | LDMR,LABD,CGRG |
| 14 | Helotrophium indicum, L. | Boraginaceae | 4.8 | + | - | - | 20 | 341 | LABS,LFSC, |
| 15 | Zizyphus rugosa Lam. | Rhamnaceae | 5.3 | + | - | - | 35 | 461 | LHTS,LFSC,CGRG |
| 16 | Justicia jendarussa L. | Acanthaceae | 6.2 | + | - | + | 26 | 375 | LAGR,LAST,GRSA |
| 17 | Solanum xanthocarpum Sch and Wendl. | Solanaceae | 5.6 | + | + | - | 47 | 572 | LCTC,LFSC,ASCB |
| 18 | Solanum nigrum L. | Solanaceae | 6.1 | + | + | - | 33 | 472 | LDMK,LFSC, |
| 19 | Plumbago auriculata Poir. | Plumbaginaceae | 6.4 | + | + | + | 24 | 143 | LABS,LHTS,LAST |
| 20 | Tragia involucrate L. | Urticaceae | 4.6 | + | - | + | 26 | 232 | LHTS,LFSC,CNGR |
| 21 | Phyllanthus amarus Schum&Thorn | Euphorbiaceae | 4.5 | + | + | - | 33 | 441 | LDMR,ASCB,CGRG |
| 22 | Jatropha curcus L. | Euphorbiaceae | 5.2 | + | + | - | 27 | 352 | LABS,LHTS,LAST |
| 23 | Thespesia populnea L. | Malvaceae | 5.3 | + | - | + | 58 | 423 | LHTS,LFSC,CNGR |
| 24 | Sida rhombifolia L. | Malvaceae | 6.3 | + | + | - | 48 | 526 | LDMR,ASCB,CGRG |
| 25 | Spillanthus calva W. | Asteraceae | 6.4 | + | + | - | 21 | 331 | LDMK,LABD,LAST |
Table 2: Isolation and identification of AM fungal species from the river bank of Bhavani, Coimbatore district, Tamil Nadu.
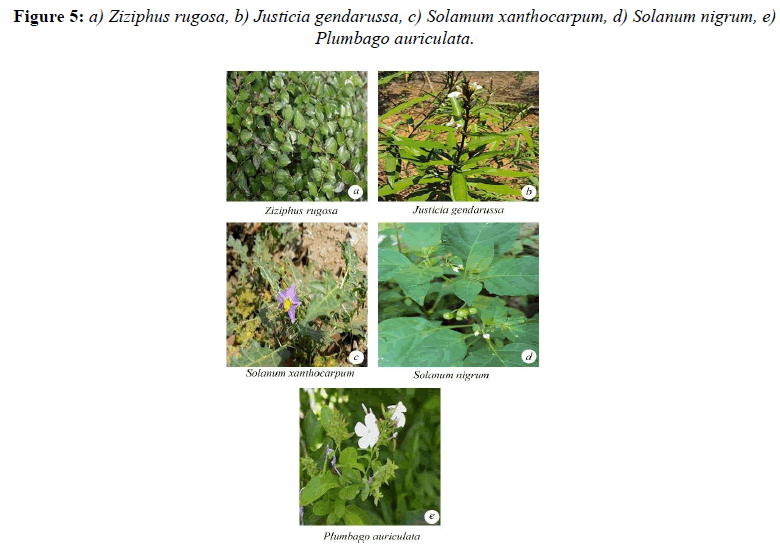
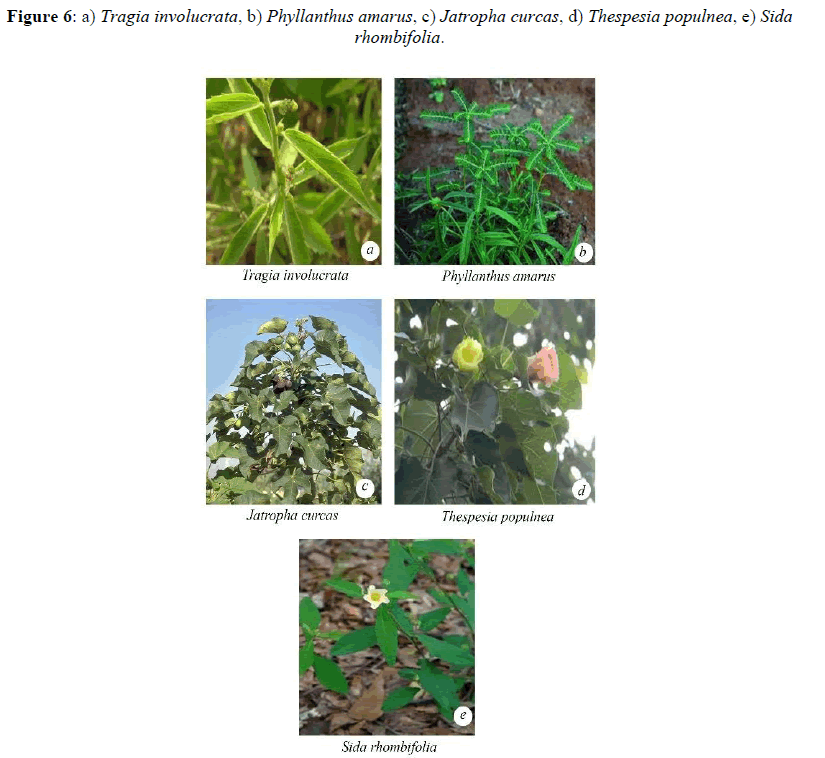
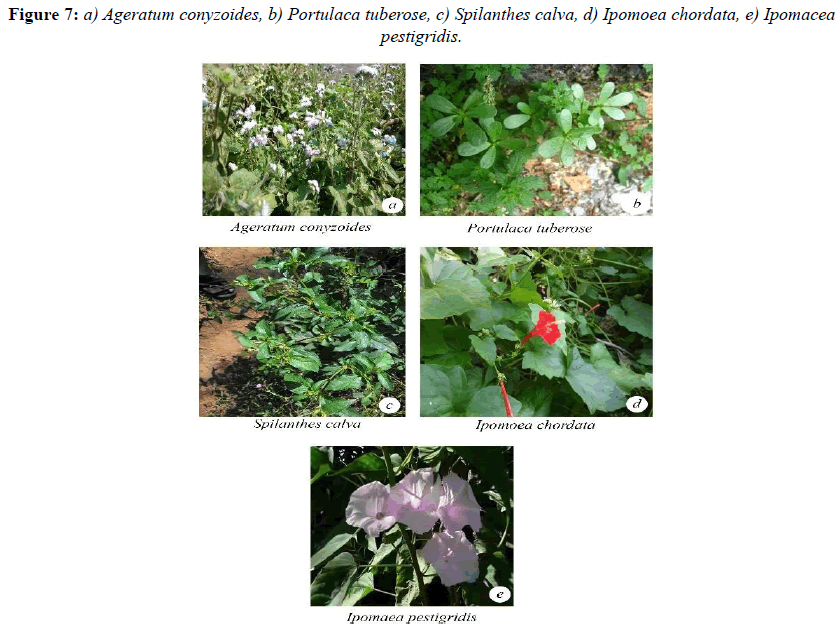
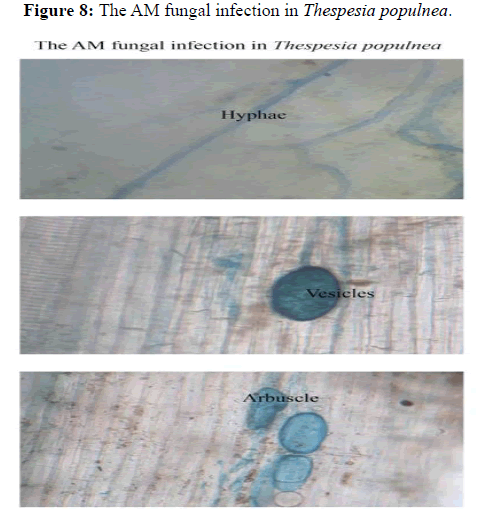
Malvaceae and the lowest was observed in Ipomea pestigridis (16%) belongs to Convolvulaceae (Figures 4-6).The species like Ludwigia parvifolora (19%) Onagraceae, Ipomea cordata (17%) Convolvulaceae showed 10 to 20% of AM fungal infection. The other species like Adathoda vasica (23%) Acanthaceae, Colachasia sp. (21%) Aeraceae, Crotalaria pallida (24%) Papilionaceae, Eragrostis umiloides (26%) Poaceae, Justicia betonica (22%)
Acanthaceae, Ipomea cordata (17%) Convolvulaceae, Portulaca tuberosa (21%) Portulacaceae, Heliotropium indicum (20%) Boraginaceae, Justicia jendarussa (26%) Acanthaceae, Plumbago auriculata (24%) Plumbaginaceae, Tragia involucrata (26%) Urticaceae, Jatropha curcus (27%) Euphorbiaceae, Spillanthes calva (21%) belongs to Asteraceae showed 20% and less than 30% of AM fungal infection.
The plant species like Ageratum conyzoides (31%) Asteraceae, Boerhavia di usa (42%) Nyctaginaceae, Cissus quadrangularis (33%) Vitaceae, Zizyphus rugosa (35%) Rhamnaceae, Solanum xanthocarpum (47%) and Solanum Sida rhombifolia (33%) Solanaceae, Phyllanthus amarus (33%) Euphorbiaceae, Sida rhombifolia (48%) Malvaceae showed 30 and less than 50% of AM fungal infection. The species like Cassia tora (53%) Caesalpinaceae showed 50 and less than 60% of AM fungal infection (Figures 7 and 8).
In the present studies, the arbuscular mycorrhizal fungal spores observed in totally 25 plant species of rhizosphere soil samples belongs to 18 plant families. Among the AM fungal species Glomus is considered to be the most common.
Figure 8: The AM fungal infection in Thespesia populnea.
All the plant species colonized by AM fungi. The plant species infected by hyphae, vesicles and arbuscules. Some of the plant species infected by only hyphal and vesicles. But all the species infected by hyphae. In the present investigation, the Euphorbiaceae family member of Pyllanthus amarus, Jatropha curcus and Ipomea pestigridis were infected by AM fungi. The same results was obtained by Pyrshangswell Kharbuli and Mishra, et al. analysed the mycorrhizal association in some trees of North-Eastern India. Majority of the plant species showed endomycorrhizal association [13]. In most of the plant species a cortical hyphae and vesicles were observed in the roots of the same plant. Mycorrhizal association occurred naturally with many important forest trees. The variation in spore population and quantum of root colonization was recorded. This fluctuation might be due to the influence of different environmental factors on AM sporulation and infection. In the present investigation, the rhizosphere soils of all the plants species contains different types of arbuscular mycorrhizal spores.
Totally 15 arbuscular mycorrhizal fungal species were isolated and identified from the river Bhavani area’s rhizosphere soil samples. The two species of Acaulospora, Aca. Appendicula, Aca. scorbuculicata one species of Gigaspora, Gig. rosea, ten species of Glomus, Gl. ambisporum, Gl. citricola, Gl. deserticola, Gl. dimorphicum, Gl. dominikki. Gl. hetrosporum. Gl.aggregatum. Gl. albidium. and Gl. fasiculatum and two species of Scutellispora, Scu. nigra and Scu. gregaria were recorded.ccname of the species with species code are presented in Table 3.
| S. no | Genus | Species | Species code |
|---|---|---|---|
| 1 | Acaulospora | Appendicula | AAPD |
| Scrobiculata | ASCB | ||
| 2 | Gigaspora | Rosea | GRSA |
| 3 | Glomus | Ambisporum | LABS |
| Citricola | LCTC | ||
| Deserticola | LDST | ||
| Dimorphicum | LDMR | ||
| Dominikii | LDMK | ||
| Heterosporum | LHTS | ||
| Aggregatum | LAGR | ||
| Albidum | LABD | ||
| Fasciculatum | LFSC | ||
| Austral | LAST | ||
| 4 | Scutellispora | Gregaria | CGRG |
| Nigra | CNGR |
Table 3: AM fungal species in river bank of Bhavani area, Mettupalayam Taluk, Coimbatore district, Tamil Nadu.
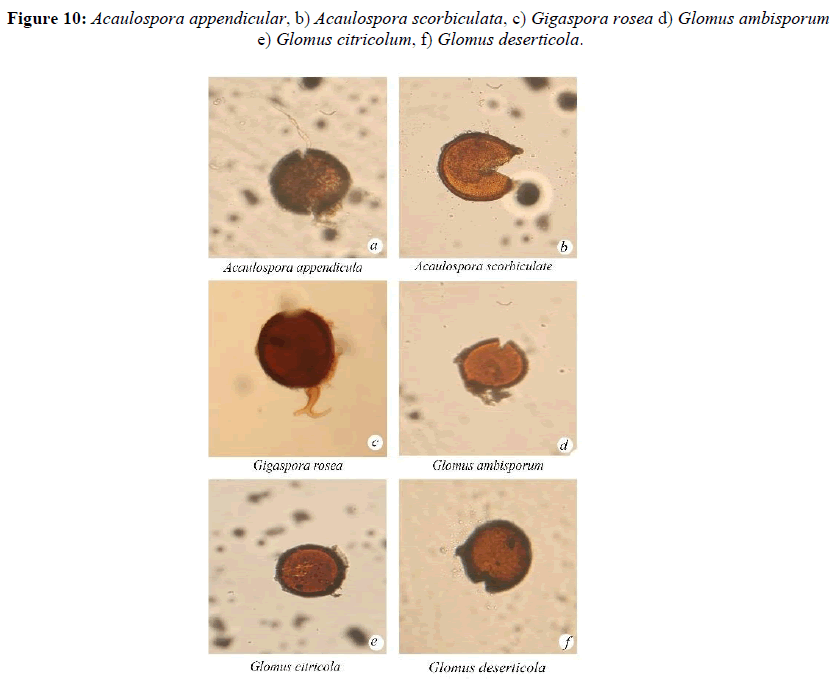
Acaulospora appendicula Spain, Sieverding and Schenck
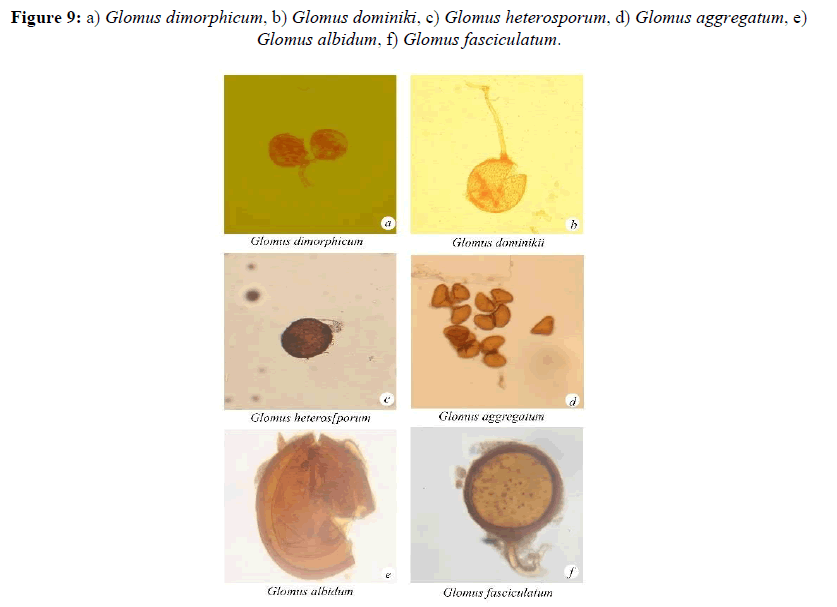
Azygospores (170-) 250 (-390) μm diam. White-opaque when young, becoming dull yellow-cream to orange-tan when mature: Hyphal pedunculate protuberance attached to the reticulate inner wall and forming an appendage on the spore. Outer wall 8-16 (-20) μm thick. Somewhat roughened. Becoming yellow to brown with age. Innermost wall hyaline smooth. 2-10 μm thick (Figure 9).
Acaulospora scorbiculata Trappe
Azygospores forming singly in the soil, sessile, borne laterallyon a wide, thin walled, hyaline hypha that terminates nearby in a thin walled vesicle. Vesicle globose, spores globose to broadly ellipsoid, olive to light brown at maturity. Spore wall continuous except at the circular, rimmed vesicle attachment ± 15 μm diam and consisting of four layers.
Gigaspora rosea, Nicolson and Schenck
Azygospores produced singly, globose, 250-300 μm diameter, cream in colour with rose-pink tint, wall upto 8 μm thick, with 2-5 inseparation layers,outer wall smooth suspensor like cell, bulbous, subtending hyphae upto 50 μm thick, wall upto 2 μm thick.
Glomus ambisporum, Smith and Schenck
Chlamydospores formed singly in the soil, sub globose, peridominatly globus and occasionally subglobose, 98-166 × 93-157 c, sporocarp dark brown to black, central core of thick interwoven hypha, periderm absent, sporocarp aggrigates, three walled spores, inner wall membranous, middle wall laminate dark brown, confluent with hyphal attachment, outer wall reticulate.
Glomus citricolum, Tang and Zang
Sporocarps unknown. Chlamydospores borne singly and also in loose clusters in the soil or in compact clusters in the cortex of roots. Spores globose to sub globose or ovoid, obovoid or irregular; 35-65 μm wide, 60-90 μm long, light brown [14]. Spore wall smooth, 2 layered. Sometimes closed distally by a septum. Forming vesicular arbuscular mycorrhizae (Figure 10).
Glomus deserticola Trappe, Bloss and Menge
Spore borne singly or in loose fascicles in soil,globose to subglobose, 54-115 × 52-102 μm, spores are shinysmooth, reddish brown in colour, with a single attached hypha 6-12 μm in diameter, cyllindric to occasionally somewhat funnel shape. Interior of the spore wall at the hyphal attachment thickened at maturity form an inner mounded color, which appears to be closed by a membranous septum.
Glomus dimorphicum, Boyetchko Tewari
Spores dimorphic, forming singly or loss clusters, single spores reddish-brown, globose to subglobose 100-250 μm three walled, 3-15 μm thick, outer wall hyaline laminated 2-7 μm thick, middle wall reddish brown 2-7 μm thick separating readily from outer wall, inner wall reddish-brown, membranous about 1 μm thick. Subtending hyphae straight or recurved, light yellow to light brown, 12-25 μm wide, wall 2-6 μm thick, point of attachment with a septum. [15].
Glomus dominiki, Blaszkowski
Chlamydospores borne singly, light yellow, ornamented, globose to sub globose, 100-175 μm diameter. Spore wall of 3 layers in 2 groups, group A with outer single wall, hyaline, 1-4 μm thick, ornamented with fine warts, group B with a hyaline, smooth, membranous <1 μm thick inner wall. Subtending hyphae hyaline, slightly recurved, up to 13 μm wide, walls 2 μm thick at the spore base, without septum, constricted at the attachment.
Glomus heterosporum, Smith and Schenck
Chlamydospores produced in Sporocarp, light to dark brown, obovoid to ellipsoid, occasionally globose, and 99-206 × 61-201 μm [16]. Spores with two distinct walls. Inner wall laminate, brown, 3-10 μm thick. Outer wall smooth, evanescent, hyaline 2-7 μm thick. Hyphae at point of attachment 5-31 μm wide. Spores frequently multiple hyphal attachments in a 43: 15:1 ratio of single dual and the triple attachments. Hyphal attachments frequently branched.
Glomus aggregatum Schenck and Smith emend.Koske
Chlamydospores formed in loose clusters, smooth, globose, to subglobose, pyriform, variable, globose spores 35-80 μm diameter, irregular spores 35-90 × 35-70 μm in size, pale yellow to orange brown, sometimes with greenish tint. Spore wall 3-5 μm thick, coloured, two separable layers, outer wall thicker. Subtending hyphae straight or curved, constricted, swollen or irregular, 5-10 μm wide.
Glomus albidum, Walker and Rhodes
Chlamydospores white, yellowish to brownish yellow, globose, spore wall 2, outer hyaline, 1-2 μm thick, inner light yellow, laminated, 1-2 μm thick, spore wall continuous with hyphal wall. Subtenting hyphae straight, 2 walled, outer wall thinckened at the spore base. Spore contents of crowded oil droplets [17].
Glomus fasciculatum, (ThaxtersensuGerd) Gerd and Trappe
Chlamydospores borne free in soil, in dead rootlets, in loose aggregations, in small compact clusters, and in sporocarps. Sporocarps up to 8 × 5 × 5 mm, irregularly globose or flattened, tuberculate, grayish brown. Peridium absent. Chlamydospores 35-105 μ diam. when globose; smooth or seeming roughened from adherent debris.
Glomus austral, (Berck) Berch
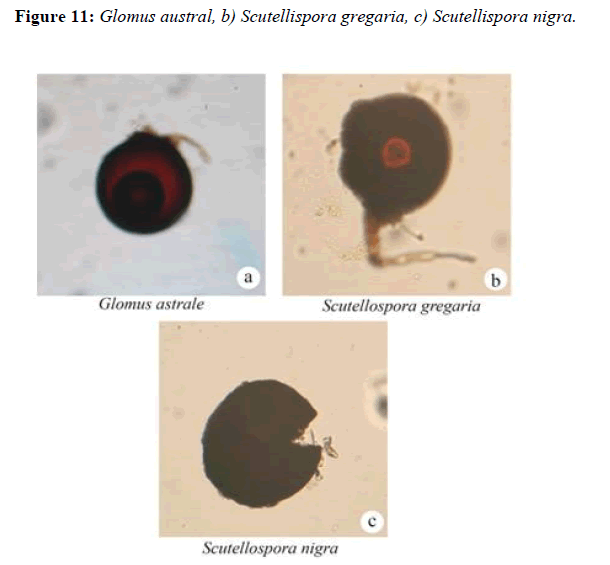
Sporocarp in reniform, epigeous, white when fresh and beige when dried. Spores forms in loose clusters which are not evident at the cut surface of the dried material. Each cluster arises from central, subtending hyphae, at the point of attachment is broad (20 μm-25 μm. Spore diameter is (120-160 (-180 μm and have two wall layers; the outer in hyaline or pale yellow, approximately 4 μm thick, the inner is light or dark brown 7 (1-15 μm thick. The spore in the subtending hyphae is often [18,19].
Scutellispora gregaria, (Schenck and Nicolson) Walker and Sanders
Spores formed singly, forcibly reddish, globose to subglobose, four cell walled 250-448 × 250-480 μm in size. Second cell brittle yellow, suspensor like cells pale brown,septate subtending hypha, thick or thin walled hypha, the suspensor cell and extending towards the spores,the large wart like projections are closely packed.
Spores formed singly, dark brown to black, globose, 2 walled, outer wall dark brown to black,pitted, inner wall light brown, transparent, laminate.Suspensor-like cell attached laterally,brown among the dark-spored species of Scutellispora [20]. Scutellispora nigra can be easily distinguished by large, black and pitted spores (Figure 11).
Conclusion
The study is undertaken to observe the Arbuscular mycorrhizal fungal association in the plants in the riverbank of Bhavani, Coimbatore district, Tamil nadu. Twenty-five plant species belongs to 18 families were analyzed for AM fungal association in the rhizosphere soil and root samples. The arbuscular mycorrizal fungal spore population and percentage of root colonization was observed in all the plant species. The mycelial structures like hyphae, arbuscules and vesicles were recorded. The soil pH was noticed in all the 25 plants species of the rhizospore soil samples.
In this study indicated that that highest AM fungal infection in Thespesia populnea (58%) and the lowest in Ipomea pestigridis (16%). The maximum spore population were reported in Cassa tora (578/100g of soil) and minimum in Colachasia sps. (121/100 g of soil).
Totally 15 AM fungal species belonging to 4 genera were observed such as Acaulospora, Gigaspora, Glomus and scuetellispora were found in the river bank of Bhavani of Coimbatore district. The two species of Acaulospora, Aca. Appendicula, Aca. scorbuculicata one species of Gigaspora, Gig. rosea, ten species of Glomus, Gl. ambisporum, Gl. citricola, Gl. deserticola, Gl. dimorphicum, Gl. dominikki. Gl. hetrosporum.
Gl. aggregatum. Gl. Albidium Gl. austral and Gl. Fasiculatum, two species of Scutellispora Scu.gregaria, Scu. nigra. Of these the Glomus genera were dominated followed by Acaulospora, Gigaspora, Scutellispora. Among the AM fungal species the Glomus fasiculatum was dominant one.
The results of the present study indicated that the AM fungal can be considered as vital diversity in the rhizosphere soils of plants species. The study concluded that the wide spread of AM fungal in the soil, and its make more favored for better growth of the plants. From the result of the present investigation, it can be concluded that there is a high incidence of AM fungi in this study area. All the plant species studied were colonized by AM fungi. AM fungi are agriculturally important and have great economic significance.
References
- Abu-Zeyad R, Khan AG, Khoo C (1999) Occurrence of arbuscular mycorrhiza in Castanospermum australe A. Cunn. & C. Fraser and effects on growth and production of castanospermine. Mycorrhiza 9:111-117
- Alexander M (1974) Introduction to soil microbiology. John Wiley and sons inc, new York. 472
- Allen MF (1991) The Ecology of Mycorrhizae. Cambridge University Press, Cambrige, UK.
- Arias I, Sainz MJ, Grace CA, Hayman DS (1987) Direct observation of vesicular-arbuscular mycorrhizal infection in fresh unstained roots. Trans Br Mycol Soc 89:128-131
- Azcon-Aguilar C, Barea JM (1997) Arbuscular mycorrhizas and biological control of soil-borne plant pathogens–an overview of the mechanisms involved. Mycorrhiza 6:457-464
- Azcon-Aguilar C, Barea JM (1997) Arbuscular mycorrhizas and biological control of soil-borne plant pathogens–an overview of the mechanisms involved. Mycorrhiza 6:457-464
- Bagyaraj DJ (1986) Mycorrhizal association in crop plants and their utilization in agriculture. Beneficial fungi and their utilization, Scientific Publication, Jodhpur 1986:59-72
- Beena KR, Raviraja NS, Arun AB, Sridhar KR (2000) Diversity of arbuscular mycorrhizal fungi on the coastal sand dunes of the west coast of India. Curr Sci 25:1459-1466
- Beena KR, Raviraja NS, Arun AB, Sridhar KR (2000) Diversity of arbuscular mycorrhizal fungi on the coastal sand dunes of the west coast of India. Curr Sci 79:1459-1466
- Bethlenfalvay GJ, Schuepp H (1994) Arbuscular mycorrhizas and agrosystem stability. Agric Ecosyst Environ 1994:117-131
- Cade-Menun BJ, Berch SM, Bomke AA (1991) Seasonal colonization of winter wheat in South coastal British columbia by vesicular–arbuscular mycorrhizal fungi. Canad J Bot 69:78-86
- Chaurasia B, Pandey A, Palni LM (2005) Distribution, colonization and diversity of arbuscular mycorrhizal fungi associated with central Himalayan rhododendrons. For Ecol Manag 207:315-324
- Copetta A, Lingua G, Berta G (2006) Effects of three AM fungi on growth, distribution of glandular hairs, and essential oil production in Ocimum basilicum L. var. Genovese. Mycorrhiza 16:485-494
[Crossref] [Google Scholar] [PubMed]
- Douds Jr DD, Millner PD (1999) Biodiversity of arbuscular mycorrhizal fungi in agroecosystems. Agric Ecosyst Environ 74:77-93
- Elsen A, Gervacio D, Swennen R, de Waele D (2008) AMF-induced biocontrol against plant parasitic nematodes in Musa sp.: A systemic effect. Mycorrhiza 18:251-256.
[Crossref] [Google Scholar] [PubMed]
- Fester T, Maier W, Strack D (1999) Accumulation of secondary compounds in barley and wheat roots in response to inoculation with an arbuscular mycorrhizal fungus and co-inoculation with rhizosphere bacteria. Mycorrhiza 8:241-246
- Gerdemann JW, Nicolson TH (1963) Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans Br Mycol Soc 46:235-244
[Crossref] [Google Scholar] [PubMed]
- Gill TS, Singh RS (2002) Effect of Glomus fasciculatum and Rhizobium inoculation on VA mycorrhizal colonization and plant growth of chickpea. J Mycol Plant Pathol. 32:162-167.
- Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytologist 84:489-500
- Govinda Rao YS, Suresh CK, Suresh NS, Mallikarjunaiah RR, Bagyaraj DJ (1989) Vesicular arbuscular mycorrhizae fungi in medicinal plants. Indian J Phytopathol 42:476-478

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences