Studies on the Antimicrobial Potency of Five Crude Plant Extracts and Chemical Fungicide in in vitro Control of Aspergillus flavus, Causal Agent of White Yam (Dioscorea rotundata) Tuber Rot
1Department of Crop and Environmental Protection, Federal University of Agriculture, PMB 2373 Makurdi, Nigeria
2Department of Crop Production and Protection, Faculty of Agriculture and Agricultural Technology, Federal University, Dutsin-Ma, PMB 5001, Katsina State, Nigeria
3Department of Crop Production Technology, Akperan Orshi College of Agriculture, Yandev, PMB 181 Gboko, Nigeria
- *Corresponding Author:
- Iorungwa Gwa
Department of Crop and Environmental Protection
Federal University of Agriculture
PMB 2373 Makurdi, Nigeria
Tel: +234 818 657 0255
E-mail: igwa@fudutsinma.edu.ng
Received date: November 11, 2016; Accepted date: November 11, 2016 Published date: December 23, 2016
Copyright: © 2016 Gwa VI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Studies on the antimicrobial potency was carried out on five crude plant extracts (Piper nigrum, Zingiber officinale, Azadirachta indica, Carica papya and Nicotiana tabacum) and a chemical fungicide (mancozeb) using three concentrations of hot aqueous plant extracts (30, 60 and 90 g/l) and mancozeb (4, 8 and 12 g/l). The extracts and chemical were separately amended in potato dextrose agar (PDA) in in vitro control of Aspergillus flavus; causal agent of yam tuber rot. Rotten white yam tubers were collected from farmers’ barns at various locations in the study area and taken to Advanced Plant Pathology Laboratory, Federal University of Agriculture, Makurdi, Nigeria for analyses. A. flavus was isolated and identified from the rotten white yam tubers; pathogenicity test was carried out to confirm the actual organism associated with the rot in yam. The test revealed that A. flavus was able to incite rot in the healthy yam tubers. The results further showed that all the test plants were able to significantly (p<0.05) inhibit the mycelia growth of A. flavus in culture. Zingiber officinale was consistently observed to be more potent irrespective of concentration and duration of incubation; this was closely followed by Piper nigrum while Azadirachta indica was third in effectiveness. The least effective plant extracts were Carica papaya and Nicotiana tabacum, respectively. The synthetic chemical, mancozeb exceedingly gave 100% inhibition of the test fungus at all the levels of concentrations and throughout the period of incubation and was statistically significant with the extracts. The findings have shown the potential of plants in the control of yam tuber rot caused by Aspergillus flavus. The use of plant products will therefore, reduce over dependence on the use of synthetic chemicals by farmers in controlling pathogens of yams as well as reducing the cost of management and environmental pollution.
Keywords
Antimicrobial; Potency; Plant extract; Fungicide; Inhibition; Yam; Aspergillus flavus
Introduction
Yam is an important crop in many parts of the tropical and subtropical regions where it is a major source of staple food for millions of people [1,2]. More than 90% of the global yam production (40 million tons fresh tubers/year) is produced in West Africa with Nigeria accounting for about 35.02 million metric tons [1]. In addition to its nutritional value, yam has considerable social and cultural significance especially among the Tivs in middle belt of Nigeria [3] and the people of South Eastern Nigeria [4]. Yams are affected by array of diseases including tuber rot caused by Aspergillus flavus in storage in yam growing areas of Nigeria.
This rotting is a major factor limiting the postharvest life of yams [5] Tuber rot organisms produce a variety of extracellular enzymes and a host of metabolites that degrade cell wall polymers, resulting in maceration of parenchymatous tissues [6]. Studies conducted in several part of the country have shown that an average of over 25% of the yield is lost annually to diseases and pests in storage [1]. Bonire estimated microbial postharvest losses in yam at 40% [7] while Okigbo and Ikediugwu indicated that between 20% and 39.5% of stored tubers may be lost to rot causing organisms [2]. Studies conducted by Arinze indicated about 50% reduction of the total stored tubers lost to rot organisms within the first 6 months of storage [8]. These make microbial tuber rot an important constraint to yam production. Most of these rots of yam tubers are caused by pathogenic fungi organisms such as Aspergillus flavus, Aspergillus niger, Botryodiplodia theobromae, Fusarium oxysporum, Fusarium solani, Penicillium chrysogenum, Rhizoctonia spp., Penicillium oxalicum, Trichoderma viride and Rhizopus nodosus [9-11]. These disease causing agents reduce both the quantity and quality of yam produced and also make them unappealing to the consumers [12]. Several control measures have been adopted for the control of yam fungal tuber rots. These include use of synthetic chemicals, biological control method and curative method as well as uses of natural plant extracts [12]. Synthetic chemicals such as sodium orthiophenylphenate, borax, captan, thiobendazole, forcelet, benomyl, nordox, bleach (sodium hypochlorite), mancozeb have been found to significantly reduce storage rot in yam [9,13,14]. However, the obvious pollution problems in the environment and the toxic effects of synthetic chemicals on non-target organisms have prompted investigations on exploiting pesticides of plant origin for control of fungal pathogens [15]. These pesticides of plant origin are specific, biodegradable, cheap, readily available and environmentally safe [16]. It is against this backdrop that the study focuses on the antimicrobial potency of some selected plant extracts against the in vitro mycelia growth of Aspergillus flavus isolated from rotten yam tubers in storage.
Material and Methods
Experimental site
The study was carried out at the Advanced Plant Pathology Laboratory, Federal University of Agriculture, Makurdi, Nigeria.
Collection of rotten yam tubers
Rotten yam tubers of white yam varieties (Dioscorea rotundata) showing various disease symptoms of dry rots were obtained from yam farmers from various storage barns in Tor- Donga, Katsina-Ala, local government area of Benue State, Nigeria which lies between longitudes 9°20' and 9°23'E, and latitude 7°17ʹ and 7°20ʹN, respectively. The rotten yam tubers were packaged in sterile polyethylene bags, taken to the laboratory for isolation and identification of pathogens. The samples were protected using wire mesh to prevent rodent attack [17]. Potato Dextrose Agar (PDA) was the medium used.
Isolation of Aspergillus flavus from rotted Yam tubers
The white yam tubers (Dioscorea rotundata) were cut from diseased and healthy parts. The cut pieces were soaked in 5% sodium hypochlorite solution for 2 minutes for surface sterilization. The pieces were then rinsed in four successive changes of sterile distilled water [18]. The yam pieces were placed on sterile paper towels in the laminar Air flow cabinet (Environmental Air control Inc. USA) to dry for 2 minutes.
Inoculation
The infected tissues were later picked onto sterile filter paper using a sterile forceps and then wrapped with filter paper for 2-3 minutes. The dried infected tissues were aseptically plated on Petri dishes containing acidified sterile potato dextrose agar (PDA) and the plates were incubated at ambient room temperature (3°C ± 5°C) for 192 hours.
Characterization and identification
Fungal colonies that grew on the incubated plates were subcultured into fresh separate sterile acidified PDA plates and incubated to obtain pure cultures of pathogens. The purified isolates were kept in slants and stored for characterization and pathogenicity test. Microscopic examination and morphological characteristics were noted and compared with existing authorities [19,20]. Aspergillus flavus which was one of the most frequently isolated organisms was selected as the test fungus.
Pathogenicity test
Healthy yam tubers were washed with running tap water, rinsed in four successive changes of sterile distilled water, Thereafter; the tubers were disinfected with 5% sodium hypochlorite solution for 30 seconds and again rinsed with sterile distilled water. The tubers were allowed to air dry. A flamed 5 mm cork borer was used to bore hole into the healthy white yam tubers [2], a 5 mm diameter disc from the pure culture of Aspergillus flavus was cut and replaced in the holes created in the healthy Dioscorea rotundata tubers..
The same procedure was used for the control except that sterile agar discs were used instead of the inoculum in the holes created in the tubers. Petroleum jelly was used to completely seal the holes [21]. The inoculated yam tubers were placed in three replications at ambient room temperature (30°C ± 5°C) under sterile condition. The plates were incubated for 14 days after which the tubers were examined for infection and disease development. The infected tubers were compared with the initially decayed tubers.
Preparation of plant extracts
Plant parts were prepared according to the methods described by [22] and [23] with some modifications. Seeds of Piper nigrum (Black Pepper), Rhizomes of Zingiber officinale (Ginger), leaves of Azadirachta indica (Neem), leaves of Carica papaya (Pawpaw) and leaves of Nicotiana tabacum (Tobacco) were washed thoroughly with cold running tab water and airdried. The dried parts of each plant species were pounded into powder separately using a sterilized mortar and pestle. Hot water (100°C) extraction was obtained by adding 30 g, 60 g and 90 g of the powder of each plant extracts to 1litre of sterile distilled water separately in 1000 ml Pyrex flask.
These were left for 24 hours and subsequently filtered through four fold of sterile cheese cloth. The filtrates obtained were used as the plant extracts in the experiment. Mancozeb was prepared in sterile distilled water at 4 g/l, 8 g/l and 12 g/l concentrations respectively. The efficacies of the aqueous plant extracts and chemical fungicide were tested in vitro for their fungicidal activity against tuber dry rot of white yam (Dioscorea rotundata) caused by Aspergillus flavus.
In vitro assay of plant extracts against Aspergillus flavus
The method of Amadioha and Obi [24] was used to determine the fungitoxic effect of different plant extracts and chemical fungicide in vitro on mycelia extension of A. flavus by creating four equal sections on each plate. This involves drawing two perpendicular lines at the bottom of the plate. The point of intersection indicates the centre of the plates. These were done before dispensing PDA into each of the plates. Then 15 ml of the prepared medium was poured into sterilized Petri dishes and 5 ml of each plant extracts and chemical fungicide at the different The method of Amadioha and Obi [24] was used to determine the fungitoxic effect of different plant extracts and chemical fungicide in vitro on mycelia extension of A. flavus by creating four equal sections on each plate. This involves drawing two perpendicular lines at the bottom of the plate. The point of intersection indicates the centre of the plates. These were done before dispensing PDA into each of the plates. Then 15 ml of the prepared medium was poured into sterilized Petri dishes and 5 ml of each plant extracts and chemical fungicide at the different levels of concentrations were poured into Petri dishes containing the media separately [25], mixed well and allowed to solidify, the solidified medium was inoculated centrally at the point of intersection of the two perpendicular lines drawn at the bottom of the plate. Five mm diameter mycelia discs retrieved from oneweek- old fresh cultures of test fungus grown on PDA plates served as inoculum [26].
The control experiments had 5 ml of sterile distilled water added to PDA in place of the plant extracts and chemical fungicide, respectively. The inoculated Petri dishes were sealed with masking tape and incubated for 120 hours at ambient room temperature (30°C ± 5°C). Radial growth of A. flavus was then recorded after every 24 hours for up to 120 hours after inoculation by measuring mycelia growth diameters along two diagonal lines previously drawn on the reverse side of each Petri dish to serve as a reference using a transparent ruler. The absence of growth in any of the plates was indicative of the potency of the extract and the chemical fungicide against the test fungus. Fungitoxicity was determined in form of percentage growth inhibition (PGI) according to the method described by Korsten and De Jager [27].
PGI (%)=(R-R1)/R*100
Where,
PGI=Percent Growth Inhibition,
R=the distance (measured in mm) from the point of inoculation to the colony margin in control plate,
R1=the distance of fungal growth from the point of inoculation to the colony margin in treated plate.
Experimental design and data analysis
The design used was Completely Randomized Design (CRD) with three replicates as described by Gomez and Gomez [28]. Test of variance was calculated using Analysis of variance (ANOVA) and statistical F-tests were evaluated at P ≤ 0.05. Differences among treatment means for each measured parameter were further separated using fishers least significance difference (LSD) to determine levels of significance according to Cochran and Cox [29].
Results
The fungus that was isolated from rotten white yam (Dioscorea rotundata) tubers resulting from the sampling survey above was identified as Aspergillus flavus. Colony characteristics growth on PDA was described as orange green in colour surrounded by a clear white zone; growth was rapid covering the entire plate within 120 hours of incubation (Figure 1).
Microscopic examination of the mycelia of the test fungus showed non-septate conidiophores arising from thick-walled foot cells. Each conidiophores ends in a terminal enlarged spherical swellings. Conidia were borne by phialides arising from a terminal swelling on the conidiophores (Figure 1). The result of Microscopic examination of the mycelia of the test fungus showed non-septate conidiophores arising from thick-walled foot cells. Each conidiophores ends in a terminal enlarged spherical swellings. Conidia were borne by phialides arising from a terminal swelling on the conidiophores (Figure 1). The result of the pathogenicity test established the susceptibility of the healthy yam tubers and invasion by Aspergillus flavus. The control experiment however, showed no rot indicating absent of the pathogen (Figure 2).
Results of the study indicated that the concentrations of the tested plant extracts against A. flavus had a positive effect in inhibiting mycelia growth in vitro. The results of A. flavus growth on PDA amended with plant extracts showed that Piper nigrum, Zingiber officinale, Azadirachta indica, Carica papaya and Nicotiana tabacum had antifungal properties against A. flavus at high concentrations (60 g/l and 90 g/l) more than at low concentration (30 g/l) in vitro (Table 1).
| Plant Extract | Concentration (g/L) | Period of Incubation (Hours) | LSD | ||||
|---|---|---|---|---|---|---|---|
| 24 | 48 | 72 | 96 | 120 | |||
| Piper nigrum | Conc I (30) | 100.00 ± 0.00a | ns79.44 ± 2.42b | b62.69 ± 4.17c | 48.79 ± 3.52d | 54.40 ± 3.29cd | 9.60 |
| Conc II (60) | 100.00 ± 0.00a | ns85.00 ± 7.64ab | ab70.56 ± 2.42bc | 54.45 ± 6.66d | 57.13 ± 2.72ccd | 15.17 | |
| Conc III (90) | 100.00 ± 0.00a | ns91.67 ± 8.33ab | a81.76 ± 2.94b | 66.48 ± 2.94c | 63.88 ± 2.68c | 13.66 | |
| LSD | - | 23.10 | 11.29 | 16.15 | 10.07 | ||
| Zingiberofficinale | Conc I (30) | 100.00 ± 0.00a | 79.44 ± 2.42b | 73.43 ± 5.50bc | 64.73 ± 2.95cd | 61.15 ± 2.57d | 10.11 |
| Conc II (60) | 100.00 ± 0.00a | 79.44 ± 2.42b | 73.89 ± 3.89b | 62.79 ± 4.96c | 62.60 ± 1.41c | 9.72 | |
| Conc III (90) | 100.00 ± 0.00a | 94.44 ± 8.56a | 81.30 ± 4.06b | 74.44 ± 4.08b | 69.01 ± 4.64b | 13.03 | |
| LSD | - | 13.04 | 15.72 | 14.12 | 10.95 | ||
| Azadiracta indica |
Conc I (30) | ns72.20 ± 14.70a | ns65.56 ± 8.68ab | ns55.56 ± 5.56ab | ns50.87 ± 2.49ab | a45.26 ± 0.89b | 25.57 |
| Conc II (60) | ns100.00 ± 0.00a | ns79.44 ± 2.42b | ns62.22 ± 6.19c | ns56.75 ± 4.29c | ab53.12 ± 4.26c | 12.65 | |
| Conc III (90) | ns100.00 ± 0.00a | ns86.11 ± 7.35b | ns70.09 ± 4.41c | ns58.84 ± 2.94c | as58.58 ± 1.52c | 12.95 | |
| LSD | 29.37 | 23.23 | 18.80 | 11.52 | 9.20 | ||
| Carica papaya |
Conc I (30) | ns55.56 ± 5.56ns | ns52.22 ± 7.78ns | ns59.72 ± 5.01ns | ns52.71 ± 4.58ns | ns48.94 ± 4.91ns | 17.91 |
| Conc II (60) | ns72.20 ± 14.70ns | ns63.30 ± 18.60ns | ns59.26 ± 9.26ns | ns54.67 ± 6.07ns | ns50.39 ± 3.46ns | 37.15 | |
| Conc III (90) | ns83.30 ± 16.70ns | ns72.78 ± 6.83ns | ns77.22 ± 6.83ns | ns62.43 ± 4.95ns | ns62.43 ± 2.95ns | 28.33 | |
| LSD | 45.76 | 42.45 | 27.05 | 18.12 | 13.36 | ||
| Nicotianatabacum | Conc I (30) | ns83.30 ± 16.70ns | ns67.22 ± 4.34ns | ns59.72 ± 5.01ns | ns54.68 ± 3.32ns | ns55.69 ± 4.08ns | 26.34 |
| Conc II (60) | ns83.30 ± 16.70ns | ns67.22 ± 4.34ns | ns59.72 ± 5.01ns | ns54.68 ± 3.32ns | ns57.13 ± 2.72ns | 26.34 | |
| Conc III (90) | ns83.30 ± 16.70ns | ns72.78 ± 6.83ns | ns63.06 ± 1.94ns | ns60.69 ± 2.45ns | ns63.88 ± 2.68ns | 26.35 | |
| LSD | 57.67 | 18.24 | 14.67 | 10.59 | 13.95 | ||
| Mancozeb® | Conc I (4) | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | - |
| Conc II (8) | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | - | |
| Conc III (12) | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | - | |
| LSD | - | - | - | - | - | ||
| Means on the same row (for each Plant Extract) with the different superscript are statistically significant (p<0.05) by period of incubation. Means on the same column (for each Plant Extract) with the different subscript are statistically significant (p<0.05) by concentration, ns=not significant. |
|||||||
Table 1 In vitro effect of different filtrate concentrations of some plant extracts and chemical fungicide at different concentrations on Percentage Growth Inhibition of Aspergillus flavus after 120 hours of incubation.
There was however, no significant difference in activity of mancozeb between concentrations I, II and III in reducing the mycelia of A. flavus in culture throughout the period of incubation (Table 1). The results also showed that the duration of incubation has a significant (P ≤ 0.05) effect on percentage growth inhibiton of A. flavus and that the potency of the extracts decreased with increase in the period of incubation (Table 2). The result further showed that Z. officinale, P. nigrum and A. indica continue to be more efficacious than C. papaya and N. tabacum at all the levels of the concentrations despite the duration of incubation.
| Plant Extract | Period of Incubation (Hours) | ||||
|---|---|---|---|---|---|
| 24 | 48 | 72 | 96 | 120 | |
| Concentration I | |||||
| Azadiractaindica | 72.20 ± 14.70ab | 65.56 ± 8.68bc | 55.56 ± 5.56c | 50.87 ± 2.49c | 45.26 ± 0.89d |
| Carica papaya | 55.56 ± 5.56b | 52.22 ± 7.78c | 59.72 ± 5.01bc | 52.71 ± 4.58c | 48.94 ± 4.94cd |
| Mancozeb® | 100.00 ± 0.00a | 100.00 ± 0.00a | 100.00 ± 0.00a | 100.00 ± 0.00a | 100.00 ± 0.00a |
| Nicotianatabacum | 83.30 ± 16.70ab | 67.22 ± 4.34bc | 59.72 ± 5.01bc | 54.68 ± 3.32c | 55.69 ± 4.08bc |
| Piper nigrum | 100.00 ± 0.00a | 79.44 ± 2.42b | 62.69 ± 4.17bc | 48.79 ± 3.52c | 54.40 ± 3.29bcd |
| Zingiberofficinale | 100.00 ± 0.00a | 79.44 ± 2.42b | 73.43 ± 5.50b | 64.73 ± 2.95b | 61.15 ± 2.57b |
| LSD | 28.81 | 16.22 | 14.27 | 9.69 | 9.66 |
| Concentration II | |||||
| Azadiractaindica | 100.00 ± 0.00 | 79.44 ± 2.42ab | 62.22 ± 6.19b | 56.75 ± 4.29b | 53.12 ± 4.26c |
| Carica papaya | 72.20 ± 14.70 | 63.30 ± 18.60b | 59.26 ± 9.26b | 54.67 ± 6.07b | 50.39 ± 3.46c |
| Mancozeb® | 100.00 ± 0.00 | 100.00 ± 0.00a | 100.00 ± 0.00a | 100.00 ± 0.00a | 100.00 ± 0.00a |
| Nicotianatabacum | 83.30 ± 16.70 | 67.22 ± 4.34b | 59.26 ± 5.01b | 54.68 ± 3.32b | 55.69 ± 4.08bc |
| Piper nigrum | 100.00 ± 0.00 | 85.00 ± 7.64ab | 70.56 ± 2.42b | 54.45 ± 6.66b | 57.13 ± 2.72bc |
| Zingiberofficinale | 100.00 ± 0.00 | 79.44 ± 2.42ab | 73.89 ± 3.89b | 62.79 ± 4.96b | 62.60 ± 1.41b |
| LSD | 27.95 | 26.19 | 16.40 | 14.62 | 9.42 |
| Concentration III | |||||
| Azadiractaindica | 100.00 ± 0.00 | 86.11 ± 7.35ab | 70.09 ± 4.41bc | 58.84 ± 2.94c | 58.58 ± 1.52c |
| Carica papaya | 83.30 ± 16.70 | 72.78 ± 6.83b | 77.22 ± 6.83b | 62.43 ± 4.95c | 62.43 ± 2.95bc |
| Mancozeb® | 100.00 ± 0.00 | 100.00 ± 0.00a | 100.00 ± 0.00a | 100.00 ± 0.00a | 100.00 ± 0.00a |
| Nicotianatabacum | 83.30 ± 16.70 | 72.78 ± 6.83b | 63.06 ± 1.94c | 60.69 ± 2.45c | 59.70 ± 3.92bc |
| Piper nigrum | 100.00 ± 0.00 | 91.67 ± 8.33ab | 81.76 ± 2.94b | 66.48 ± 2.94bc | 63.88 ± 2.68bc |
| Zingiberofficinale | 100.00 ± 0.00 | 94.44 ± 5.56a | 81.30 ± 4.06b | 74.44 ± 4.08b | 69.01 ± 4.64b |
| LSD | 29.65 | 19.79 | 12.26 | 10.10 | 9.34 |
| Means on the same column (for each concentration) with different superscript are statistically significant (p<0.05). (Conc I=30g/L of Plant extract, 4 g/l of Mancozeb; Conc II=60g/L of Plant extract, 8 g/l of Mancozeb; Conc III=90g/L of Plant extract, 12 g/l of Mancozeb). | |||||
Table 2 In vitro Percentage Growth Inhibition of Aspergillus flavus by some plant extracts and chemical fungicide at different concentrations after 120 hours of incubation.
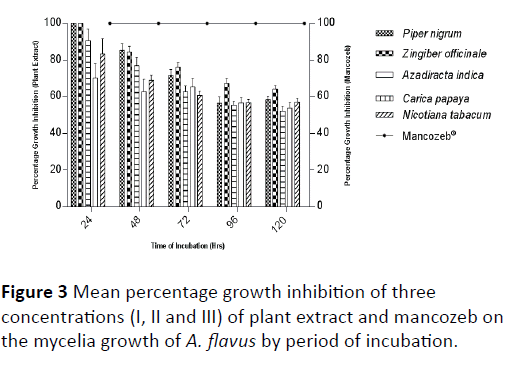
Though, the potency of each plant extract decreased with prolonged incubation period, N. tabacum was considered more potent at concentration I and II more than C. papaya while at concentration III C. papaya had a higher percentage growth inhibition than N. tabacum. The synthetic fungicide, mancozeb which showed the highest percentage growth inhibition irrespective of the concentration was considered more effective in reducing the mycelia growth of A. flavus than the plant extracts and also showed significant difference (P ≤ 0.05) with the plant extracts at all the levels of concentrations and throughout the period of incubation (Tables 2 and 3). Mean percentage growth inhibition of three concentrations (I, II and III) after 120 hours of incubation showed that Z. officinale, P. nigrum and A. indica were more effective in reducing the mycelia growth of A. flavus in culture while the least effective plant extracts were C. papaya and N. tabacum respectively (Figure 3).
| Plant Extract | Concentrations | ||
|---|---|---|---|
| Conc I | Conc II | Conc III | |
| Azadiractaindica | 57.89±4.03d | 70.31±4.89bc | 74.73±4.59bcd |
| Carica papaya | 53.83±2.36d | 59.98±4.89c | 71.64±4.05cd |
| Mancozeb® | 100.00±0.00a | 100.00±0.00a | 100.00±0.00a |
| Nicotianatabacum | 64.13±4.24cd | 64.13±4.24bc | 67.91±3.97d |
| Piper nigrum | 69.06±5.11bc | 73.43±4.94b | 80.76±4.08bc |
| Zingiberofficinale | 75.75±3.86b | 75.74±3.86b | 83.84±3.50b |
| LSD | 10.32 | 11.76 | 10.41 |
| Means on the same column with the different superscript are statistically significant (p<0.05). (Conc I=30g/l of Plant extract, 4 g/l of Mancozeb; conc II=60g/l of Plant extract, 8 g/l of Mancozeb; Conc=90g/l of Plant extract, 12 g/l of Mancozeb). | |||
Table 3 Mean percentage growth inhibition of Aspergillus flavus by some plant extracts and chemical fungicide at different concentrations after 120 hours of incubation.
Discussion
The study has shown that Aspergillus flavus is a major rot pathogen of yam in Tor-Donga which corroborates the results earlier on reported by Ogunleye and Ayansola [10] and Okigbo et al. [11] in other parts of Nigeria. The study revealed that fungitoxic compounds were present in Z. officinale, P. nigrum, A. indica, C. papaya and N. tabacum since they were able to suppress the growth of A. flavus tested. This agrees with earlier reports by some workers on the effect of these plants on pathogens of some crops [30,31]. Onifade also reported the control of C. lindemuthianum using A. indica seed; leaf, bark and root extract, recording a 100% inhibition of spore germination and mycelial growth [32]. Ejale and Abdullah indicated that the dried leaf powder of A. indica was used to control the postharvest rot of tomato [33]. On the other hand, Oluma and Elaigwe observed that extracts of A. indica had no inbibitory effect on the mycelial growth and sclerotial formation of Macrophomina phaseolina [34]. Biu et al., in his investigation observed the presence of anti-nutrients like saponins, tannins, glycosides, alkaloids, terpenes and flavenoids in the aqueous extracts of the leaves of Azadirachta indica [35]. Hycenth reported the antifungal effect of Azardirachta indica against yam rot pathogens (Rhizopus stolonifer) [36]. Taiga et al. showed that N. tabacum cold extract inhibited the Mycelia of F. oxysporum yam rot organism [22]. Amienyo and Ataga used Z. officinale, Annona muricata, Gacinia cola, Alehornea cordifolia, Allium sativum to control wet rot on sweet potatoes caused by rot fungal pathogens [21]. The antifungal properties of Z. officinalis on Aspergilus flavus, Aspergilus niger, Fusarium solani and Fusarium oxysporum on post-harvest yam (Dioscorea alata, Poir) has been reported by Yeni [37]. Shiva et al. evaluated the antimicrobial activity of piperine against Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Escherichia coli, Alternaria alternata, Aspergillus niger, Aspergillus flavus and Fusarium oxysporum and found out that the active ingredient in Piper nigrum has inhibitory effect on these pathogens [31]. Ebele used C. papaya; C. adorata and Acalypha ciliata to control pawpaw fruit rot fungi [38]. Ogwulumba et al. used Carica papaya leaf extracts to control incidence of foliar mycopathogens of groundnut (Arachis hypogea) [39] while Suleiman, inhibited mycelia growth of Alternaria solani, causal organism of yam rot using leaf extracts of Carica papaya [30]. Assessment of the effect of mancozeb on Aspergillus flavus mycelia showed that increase in the concentration of mancozeb and duration of incubation does not affect growth inhibitions as the test fungus had already attained the highest level of inhibition (100%) at the lowest concentration (4 g/l). This result agreed with the report of Emua and Fajola, who found out that mancozeb consistently gave 100% inhibition (at concentrations of 250 ppm, 500 ppm and 1000 ppm) of germination of conidia of Cercospora contraria and Didymosphaeria donacina which caused leaf spot diseases of cluster yam (Dioscorea dumetorum) [40].
On the other hand, Amadioha and Markson observed that increase in the concentration of the chemicals and duration of incubation positively correlated with the growth inhibitions [13,41]. The variation noted in the antimycotic effects of the extracts may be as a result of solubility of the active substances in water or the presence of inhibitor against fungicidal principles. This is in agreement with the investigations of Amadioha [42].
Conclusion
The findings have shown the potential of plants in the control of yam rotting fungus caused by Aspergillus flavus. The result revealed that plant extracts could be an alternative to toxic fungicides for controlling plant pathogens since they are composed of various bioactive compounds such as alkaloids, flavonoids, glycosides, phenols, saponins, sterols, etc. Lakshmeesha et al. [43]. The use of plant products will therefore, reduce over dependence on the use of synthetic chemicals by farmers in controlling yam fungal pathogens as well as reducing cost of management and environmental pollution.
References
- https://faostat.org.
- Okigbo RN, Ikediugwu FEO (2000) Studies on Biological Control of Post-Harvest Rot in Yams (Dioscorearotundata) using Trichoderrmaviride, J Phytopathol 148: 351-355.
- Orkwor GC (1992) Yams production, cropping systems, ecology, Development and Research in Nigeria, Paper presented at the National seed and plant quarantine project, Nigeria.
- Okigbo RN, Anukwuorji CA, Ezeabara CA (2013) Effects of leaf extracts of Chromolaenaodorata (L.) and Terminaliacatappa (L.) on mycelia growth of yam tuber pathogens. Niger. J PltProt 27: 74-84.
- Osagie AU (1992) The yam tuber in storage. Postharvest research Unit, Department of Biochemistry, University of Benin, Benin-City, Nigeria 146-149.
- Ikotun T (1984) Production of oxalic acid by Penicilliumoxalicum in culture and infected yam tissue and interaction with macerating enzyme. Mycopathologia 88: 9-14.
- Bonire JJ (1985) Preventing yam rot with organotin compounds. Nigeria J Sci 19:145-148.
- Arinze AE (2005) Plant Pathology and Post–harvest Food Loss. An Inaugural Lecture Series 43:29-72.
- Aidoo KA (2007) Identification of yam tuber rots fungi from storage systems at the Kumasi Central market. A dissertation submitted to Faculty of Agriculture, K.N.U.S.T.
- Ogunleye AO, Ayansola OT (2014) Studies of Some Isolated Rot-Causing Mycoflora of Yams (Dioscorea Spp.). Amer J MicrobBiot 1: 9-20.
- Okigbo NR, EnweremaduCE, Agu CK, Irondi RC, Okeke BC, et al. (2015) Control of white yam (Dioscorearotundata) rot pathogen using peel extract of water yam (Dioscoreaalata). AdvApplSci Res 6: 7-13.
- Amusa NA, Adegite AA, Mohammed S, Baiyewu RA (2003) Yam disease and its management in Nigeria. AfrJ Biotechnol 2: 497-502.
- Markson AA, Amadioha AC, Omosun G, Madunagu BE, Udo SE, et al. (2012) Control of Botryodiplodiatheobromae causing Tissue Rot of White Yam (DioscorearotundataPoir) Scholarly JAgricSci 2: 1-7.
- Nnodu EC, Nwankiti AO (1986) Chemical control of post-harvest deterioration of yam tubers. FitopatologiaBrasileira 1: 865-871.
- Amadioha AC, Markson AA (2007a) Post harvest control of tuber rot by Botryodiplodiaacerina using extracts of plant origin. Archives of Phytopathol and Protection 40: 359-366.
- Okigbo RN, Nmeka IA (2005) Control of yam tuber with leaf extracts of Xylopiaaethiopica and Zingiberofficinale. Afr J Biotech 4: 804-807.
- Eze CS (1984) Studies on storage rot of cocoyam (Colocasiaesculenta (L.) Schott) at Nsukka. MSc Dissertation, Dept of Botany, Univ of Nigeria, Nsukka, p:73.
- Ritchie B (1991) Practical techniques in plant pathology CAB. Wallingford, UK.
- Ahmed KM, Ravinder Reddy Ch (1993) A pictorial guide to the identification of seed borne fungi of sorghum, pear millet, finger millet, chickpea, pigeonpea and groundnut.Information Bulletin no. 34: (In En summaries in Fr, Es and Ar.) Patancheru, A. P. 502324, India: Internation Crops Research Institute for the Semi-Arid Tropics,pp:200.
- Burgess LW, Knight TE, Tesoriero L, Phan HT (2008) Diagnostic manual for plant diseases in Vietnam. ACIAR Monograph No. 129, ACIAR: Canberr,p:210.
- Amienyo CA, Ataga AE (2007) Use of indigenous plant extracts for the protection of mechanically injured sweet potato (Ipomeabatatas (L.) Lam) tubers Scientific Research and Essay 2:167-170.
- Taiga A, Suleiman MN, Sule W, Olufolaji DB (2008) Comparative in vitro inhibitory effects of cold extracts of some fungicidal plants on Fusariumoxysporium Mycelium 7: 3306-3308.
- Tijjani A, Adebitan SA, Gurama AU, Aliyu M, Dawakiji AY, Haruna SG, Muhammmed NA (2013) Efficacy of Some Botanicals for the Control of Wet Rot Disease on Mechanically Injured Sweet Potato Caused by RhizopusStolonifer in Bauchi State Int J Sci Res Pub 3:1-10.
- Amadioha AC, Obi VI (1999) Control of anthracnose disease of cowpea Cymbopogoncitratus and Ocimumgratissimum. ActaphytopatholEntomolHungerica 34: 85-89.
- Nene ZH, Thapilyal (2002) Management of mushroom pathogens through botanicals. Ind. Phytopathol. 58: 189-193.
- Vedashree S, Sateesh MK, Lakshmeesha TR, Sofi MS, Vedamurthy AB (2013) Screening and assay of extracellular enzymes in Phomopsisazadirachtae causing die-back disease of neem. J Agricultural Technol 9: 915-927.
- Korsten L, De Jager ES (1995) Mode of action of Bacillus subtilis for control of avocado post-harvest pathogens. S. Afr. Avocado Growers Assoc. Yearb 18: 124-130.
- Gomez KA, Gomez AA (1984) Statistical procedures for Agricultural Research, 2nd Edn, John Wiley and Sons,p: 680.
- Cochran GW, Cox GM (1992) Experimental Designs. 2nd Edn John willey and Sons Inc., p: 611.
- Suleiman MN (2010) Fungitoxic Activity of Neem and Pawpaw Leaves Extracts on AlternariaSolani, Causal Organism of Yam Rots: Adv. Environ. Biol. 4: 159-161.
- Shiva RSK, Neeti S, Udaysree (2013) Antimicrobial Activity of Black Pepper (Piper nigrum L.).Global J Pharmacol 7: 87-90.
- Onifade AK, (2002) Antifungal effect of Azadirachtaindica A. Juss extracts on Collectotricumindemathianum. Global J Pure ApplSci 6: 423-428.
- Ejale AU, Abdullah HO (2004) Preservation of ripe tomato fruits with dried leaf powder of Neem. Nigerian J ApplSci 22: 344-370.
- Oluma HOA, Elaigwe M (2006) Antifungal activity of extracts of some medicinal plants against Macrophominaphaseolina. J Bot 19: 121-28.
- Biu AA, Yusuf SD, Rabo JS (2009) Phytochemical screening of Azadirachtaindica (Neem). Biosci Res Commun 21: 281-283.
- Hycenth N (2008) Effect of different plant extracts in the control of yam (Dioscoreasp) in Yola, Adamawa state, Nigeria. NigeriAgric J 3: 382-387.
- Yeni IJ (2011) Evaluation of Antifungal Effects of Extracts of Allium sativum and Nicotianatabacum against Soft Rot of Yam (Dioscoreaalata). J Agric Res 3: 1-5.
- Ebele MI (2011) Evaluation of some aqueous plant extracts used in the control of pawpaw (Carica papaya L.) Fruits rot fungi. J ApplBiosci 37: 2419-2424.
- Ogwulumba SI, Ugwuoke KI, Iloba C (2008) Prophylactic effect of pawpaw leaf and bitter leaf extracts on the incidence of foliar mycopathogens of groundnut (Arachishypogaea L.) in Ishiagu, Nigeria. Afr J Biotechnol 7:2878-2880.
- Emua SA, Fajola AO (1983) Chemical control of two leaf spot diseases of cluster yam (Dioscoreadumetorum) caused by Cercosporacontraria and Didymosphaeriadonacina. Plant Dis 67: 389-391.
- Amadioha AC (2003) Evaluation of some Plants leaf extracts against Colletotrichumlindemuthianum in Cowpea. ActaPhytopathologia et EntomologicaHungarica 38: 259-265.
- Amadioha AC (2000) Fungitoxic effect of some of some extracts against Rhizopusoryzae causing rot of potato. Archives of Phytopath Plant Protect 33: 499-507.
- Lakshmeesha TR, Sateesh MK, Vedashree S, Sofi MS,Umesha S (2013a) Efficacy of botanicals on soybean seed-borne Fusariumequiseti. VCFL Sci 3: 10-16.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences