ISSN : 0976-8505
Der Chemica Sinica
Structural Characteristics and Fibrinolytic Property of Rediscovering Rare Compounds from Polar-Derived Strain of Marine Fungi Geomyces Pannorum
Guo R Hua1,2,4 Duan Dong1,2,4, Wang S Jun3, Fu Qiang1,2,4,Bao Bin1* and WU W Hui1,2,3,4*
1Department of Marine Pharmacology, College of Food Science and Technology, Shanghai Ocean University, Shanghai, China
2Shanghai Engineering Research Center of Aquatic-Product Processing & Preservation, Shanghai, China
3Co-Innovation Center of Jiangsu Marine Bio-industry Technology, Huaihai Institute of Technology, Lianyungang, China
4Laboratory of Quality and Safety Risk Assessment for Aquatic Products on Storage and Preservation (Shanghai), Ministry of Agriculture, Shanghai, China
- *Corresponding Authors:
- Bao Bin
Department of Marine Pharmacology
College of Food Science and Technology
Shanghai Ocean University
Shanghai, China - WU W Hui
Department of Marine Pharmacology
Laboratory of Quality and Safety Risk Assessment for Aquatic Products on Storage and Preservation (Shanghai)
Ministry of Agriculture, Shanghai, China
Abstract
In the present study, fibrinolytic compounds were screened from marine microorganisms. Fungi and actinomycetes, Geomyces sp., Penicillium sp., and Aureobasidium pullulans, etc, were evaluated for their effects on plasminogen using a natural marine fibrinolytic compound FGFC1 as a positive control. It is the first report that Geomyces pannorum has the fibrinolytic activity in vitro. Six compounds of metabolites from Geomyces pannorum, tridec-1-ene (1), methyl (5Z,9Z)-octadeca-5,9-dienoate (2), (E)-icos-4-ene (3), hexyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl) propanoate (4), 5α,8α-epidioxy ergosta-6,22-diene-3β-ol (5) and dibutyl phthalate (6), were isolated and their structures were elucidated by 1H NMR, 13C NMR, 1H–1HCOSY, HMBC, HMQC and MS spectrum. They were identified as long chain fatty acid, sterol and aromatic hydrocarbon, whose fibrinolytic activities were estimated. The results showed that rediscovering rare compounds 2, 3, 5 and 6 could potentially activate the plasminogen and it also showed moderate fibrinolytic activities in vitro with EC50 values of 120.0, 181.3, 200.0 and 179.7 μg/mL, respectively. Accordingly, the results disclosed that Geomyces sp. might have abundant and cultivable microorganisms with potential metabolites for antithrombotic drugs application in biopharmaceutical industries.
Keywords
Microbial metabolites, Fibrinolysis, Lead compound, Geomyces pannorum, FGFC1
Introduction
A large number of bioactive compounds have been identified from marine resources. Whereas, compounds from marine fungi were not completely characterized still [1]. Numerous studies showed that marine microorganisms have various biological functions including neuroprotective effects [2], antimicrobial4 [3,4] and anticancer properties [5], antioxidant [6] and anti-inflammatory [7,8]. Accumulation of fibrin in the blood vessels usually leads to thrombosis, resulting in cardiovascular diseases which are the leading causes of death worldwide [9]. However, typical thrombolytic agents like t-PA, urokinase-type plasminogen activator (u-PA), and streptokinase (SK) aren’t satisfactory due to several disadvantages such as low specificity for fibrin, a short half-life, and relatively expensive prices, especially haemorrhage risks [10]. Hence, researchers are searching for small molecules with antithrombotic activities. In previous study, we reported that marine fungi fibrinolytic compound FGFC1 [11], 1-O-Palmitoyl-2-O-oleoyl-3-O- (α-D-glucopyranosyl)-glycerol (POGG) [12], and FGFC2 [13] showed significantly antithrombotic properties. In our continuing research on active leads from metabolites of marine organism, we investigated metabolites of 111 microorganism strains, and evaluated their fibrinolytic activities. The results showed that the extract of Geomyces pannorum had significant fibrinolytic activity in vitro.
To date, the genus Geomyces includes only nine known species [14]. Most researches focused on white-nose syndrome (WNS) caused by Geomyces Destructans [15]. To our knowledge, only several compounds with antimicrobial activity from Geomyces strains of terrestrial origin have been described. A kind of bioactive asterric acid isolated from the Antarctic ascomycete fungus Geomyces sp. has been described, of which metabolite geomycins displayed antifungal and antimicrobial activity against Aspergillus fumigatus [16]. A lipase, LipG7 purified from the Antarctic filamentous fungus Geomyces sp., was found to have industrial potential as an enantioselective biocatalyst as it is able to catalyze effectively the enantioselective trans esterification of a secondary alcohol [17]. As for G. pannorum, only one compound pannomycin with weak antibacterial activity, cis-decalin structure, has been described [18]. On contrary, antithrombotic activities and compounds were not reported from G. pannorum.
In this paper, further investigations on metabolites of G. pannorum led to the isolation of six compounds, tridec-1- ene (1), methyl (5Z,9Z)-octadeca-5,9-dienoate (2), (E)-icos-4-ene (3), hexyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl) propanoate (4), 5α,8α-epidioxy ergosta-6,22-diene-3β-ol (5) and dibutyl phthalate (6). Their structures were elucidated by comprehensive spectroscopic analysis including 1H NMR, 13C NMR, 1H–1HCOSY, HMBC, HMQC and MS. The assays of fibrinolytic activity indicated that six compounds exhibited fibrinolytic activity. Compared with positive control FGFC1, the six compounds showed moderate fibrinolytic effects with EC50 values of 120.0, 181.3, 200.0 and 179.7 μg/mL, respectively.
A research on therapeutic lead molecules encompasses many steps to more efficient and available compounds. The principle are general rules. This study intends to cover processes in molecular structures of different scaffolds that include the discovery of new or rare compounds for fibrinolysis, the traditionally unknown bioactive natural products by screening and marine fungi cultures from polar-derived strain. The study has also included the marine natural products by simple purified methodologies.
Materials and Methods
General methods and materials
Strains were collected from the South Polar Sea sand storage by China Center for Type Culture Collection (CCTCC). Experimental strains were cultured with yeast-maltose medium (YM medium) containing water 1.0 L, yeast extract (3.0 g), wort agar (3.0 g), peptone (5.0 g), glucose (20.0 g), and fermented using Chavez medium containing NaNO3 (3.0 g), K2HPO4•3H2O (0.13 g), KCl (0.5 g), MgSO4 (0.5 g), FeSO4 (0.015 g), sucrose (50 g), yeast extract (1.0 g), CoCl2 (0.0025 g), CaCl2 (0.0065 g), arginine (5.0 g), L-ornithine (5.0 g), and water (1.0 L). The chemical solvent using methanol, dichloromethane, acetone, ethyl acetate and petroleum ether were AR grade, which were purchased from Sino pharm Chemical Reagent Co. Ltd, Shanghai, China. L-ornithine purchased from Shanghai Yuanye Biotechnology Co. Ltd., Shanghai, China. Single chain urokinase-type plasminogen activator (Pro-uPA), plasminogen (Plg) and bull serum albumin (BSA) were purchased from Sigma-Aldrich, Shanghai, China. Chromogenic substrate for plasmin S-2444 purchased from Shanghai Boatman Biotech Co. Ltd. 1HNMR (500 MHz, CDCl3) and 13CNMR (125 MHz, CDCl3, Bruker BioSpin GmbH) was carried out by Jiangsu Marine Bio-industry Technology, Huaihai Institute of Technology, Lianyungang, China.
Screening of fibrinolytic substances from marine microorganisms
A total of 111 strains including fungi and actinomycetes, such as Geomyces sp., Penicillium sp., and Aureobasidium pullulans, were activated using medium at 180 rpm for 3 d, and then fermented using Chavez medium at 180 rpm for 7 d (pH 5.8). Initial inoculum added 5% L-ornithine. Then 2.0 mL fermentation broth of organisms were extracted with methanol (5.0 mL, 3 times), centrifugation and supernatant drying by vacuum dryer and re-dissolved in 1.0 mL 0.05 mol/L Tris-HCl buffer solution containing NaCl (100 mmol/L) at pH 7.4. BSA (10 μL, 10 mg/mL), plasminogen (10 μL, 50 nmol/L), S-2444 (10 μL, 2 mmol/L), Pro-uPA (10 μL, 100 nmol/L), and 10 μL sample (control add 10 μL Tris- HCl buffer) were added and incubated in 96-well clear polystyrene micro plates (Corning) test in ELISA (Corona, SH-1000LAB) at 37℃ for 150 min. The rate of fibrinolytic activity by plasmin generation was calculated according to the absorbance at 405 nm (A405) versus time squared (t2) plot slopes.
Extraction, purification of fibrinolytic organic substances
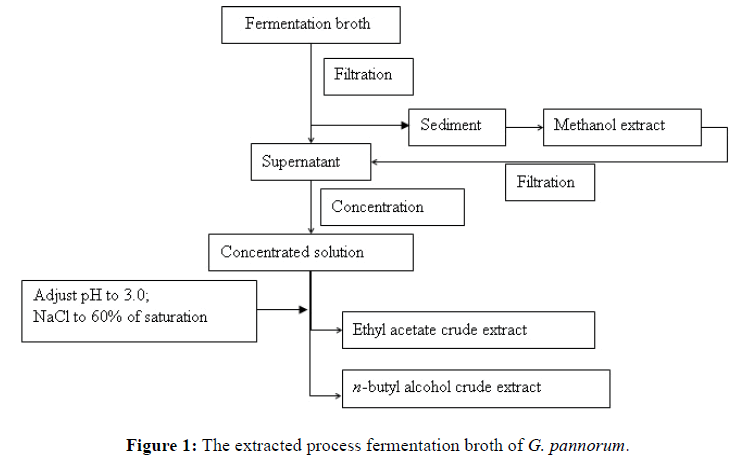
The fermentation broth of G. pannorum was centrifuged at 10,000 rpm for 10 min, using High-speed Refrigerated centrifuge (HITACHI, CR 21G, Japan). Sediments were extracted with methanol (200 mL, ultrasonic for 10 min, 3 times) and filtered. The methanol extracts and centrifugal supernatant were combined, concentrated under rotary evaporator at 45℃. Concentrated solution was adjusted pH to 3.0, added NaCl to 60 % of saturation, extracted with ethyl acetate (500 mL, 3 times), and the resultant organic fraction was dried by aqueous Na2SO4 for dehydration and then concentrated under rotary evaporators at 45℃ (Figure 1). The ethyl acetate extract were fractionated on a silica gel C18 (200-300 mesh, Qingdao haiyang chemical Co., Ltd.) flash column using column chromatography and gradient elution (dichloromethane: methanol: water, 50:1 → 4:6:0.1, V/V/V), then four fractions were obtained. Furthermore, fraction I (310.6 mg) was purified by silica gel column and eluted with petroleum ether: ethyl acetate (50:1, V/V) to product compounds 1 and 2. Fraction (286.5 mg) was subjected to column chromatography (silica gel, dichloromethane: ethyl acetate, 100:1, V/V) to obtain compounds 3 and 4. Fraction III (203.1 mg) was eluted with dichloromethane: acetone (20:1, V/V) on column silica gel chromatography to yield compound 5. Fraction IV (223.1 mg) was subjected to column chromatography (sfilica gel, ethyl acetate: acetone, 15:1, V/V) eluted with ethyl acetate: acetone (15:1, V/V) to yield compound 6.
Evaluation of isolated compounds in-vitro
The assay of fibrinolytic activity was performed using a modified fibrin plate method [19], which is suitable for small molecular compounds and contains bovine plasminogen, single chain urokinase type plasminogen activator (pro-uPA) and purified compounds as a contamination. Purified compounds dissolved in 0.05 mol/L Tris-HCl buffer containing NaCl (100 mmol/L) at pH 7.4 and the BSA used as a substrate. After adding the samples, pro-uPA (20 μmol/L) was added into the 96-well microplate. The microplate was then incubated at 37℃ for 150 min. The continuous variation trend of absorbance was determined for evaluation of fibrinolytic activity on the slope of the plots of A405 nm. Marine natural fibrinolytic compound FGFC1 was used as a positive control.
Results and Discussion
Screening of strains for fibrinolytic activity
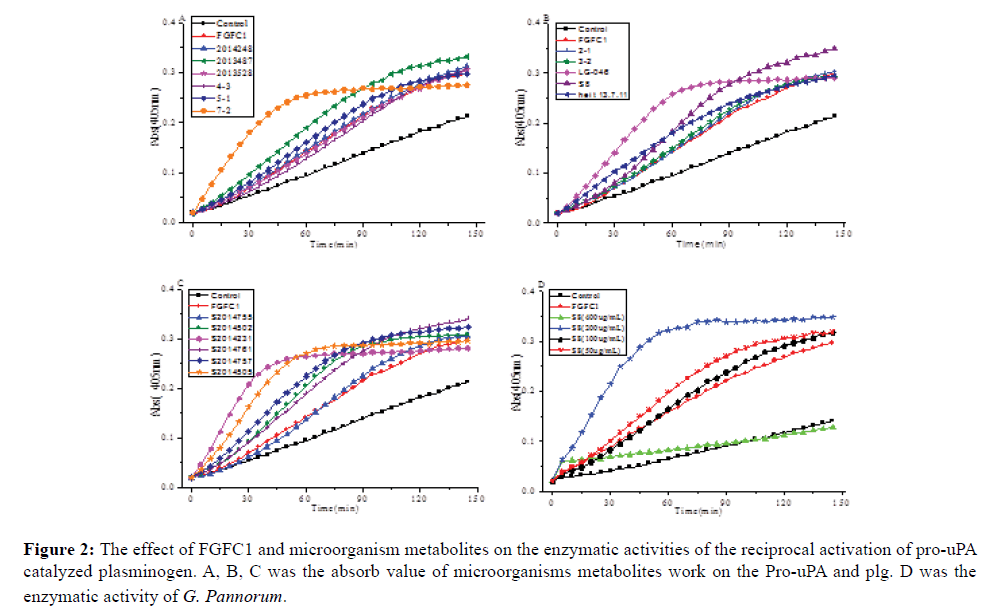
A total of 111 polar marine microorganisms broth were tested for their fibrinolytic activity. Crude extracts and organic fractions of strains were obtained after growth in Chavez medium. Samples were evaluated for fibrinolytic activities assays. Rhodotorula sp (7-2), Penicillium cavernicola (S2014505), G. pannorum (S8), and Aureobasidium proteae (S2014231) of seventeen strains showed promising result by exhibiting significant promote activities on plasminogen in vitro (Figure 2A-2C). The results revealed that polar marine have abundant and cultivable microorganisms with potential metabolites for antithrombotic drugs application.
Figure 2: The effect of FGFC1 and microorganism metabolites on the enzymatic activities of the reciprocal activation of pro-uPA catalyzed plasminogen. A, B, C was the absorb value of microorganisms metabolites work on the Pro-uPA and plg. D was the enzymatic activity of G. Pannorum.
S8 (G. pannorum) were screened out for its obviously fibrinolytic activities at the dilution ratio of 200 μg/mL concentration in vitro (Figure 2D) and cultivable growth condition. Compared to FGFC1 as a reference drug, the extract broth of G. pannorum showed stronger activity.
Growth conditions of G. pannorum
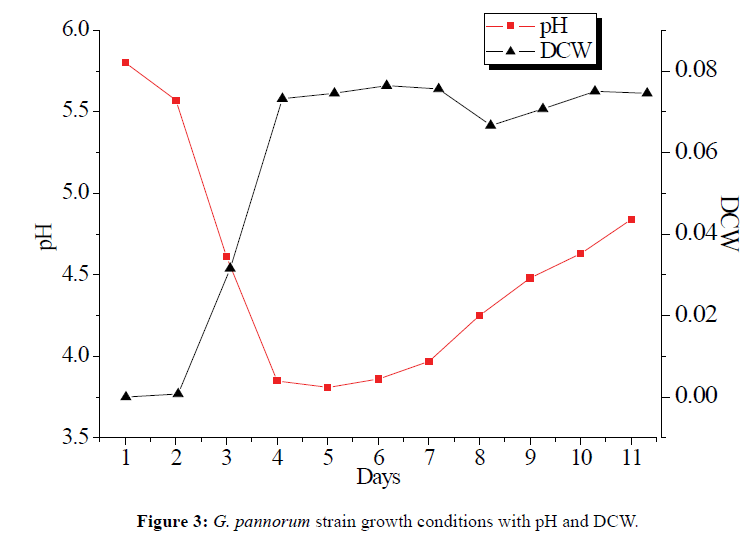
The fermented process of marine microorganisms is not entirely random and metabolites are changed by the fermentation condition [20]. The most important fact of growth condition was pH and medium, feeding of limiting metabolic precursors, which can help to identify the biosynthetic pathway of the metabolite and enhance the production of secondary metabolites [21]. The growth of G. pannorum included four stages in the fermented process. Phase I (1-2 d, adaptive phase): The dryer cell weight (DCW) increased slowly, and pH decreased slightly. Phase II (2-5 d, growth phase): pH decreased rapidly at this phase, and mycelia increased drastically and stayed at the highest level. Phase III (5-7 d, stable phase): pH began to increase with the balance of mycelia autolysis and growth. Phase IV (after 7 d): pH growth and mycelia autolysis decreased rapidly. The growth improved when the medium containing 1% L-ornithine (5.0 mL) for each bottle at the seventh day (Figure 3). Abundant metabolite levels were stimulated when G. pannorum growes on Chavez medium with superfluous L-ornithine and sucrose.
Identification of the constituents
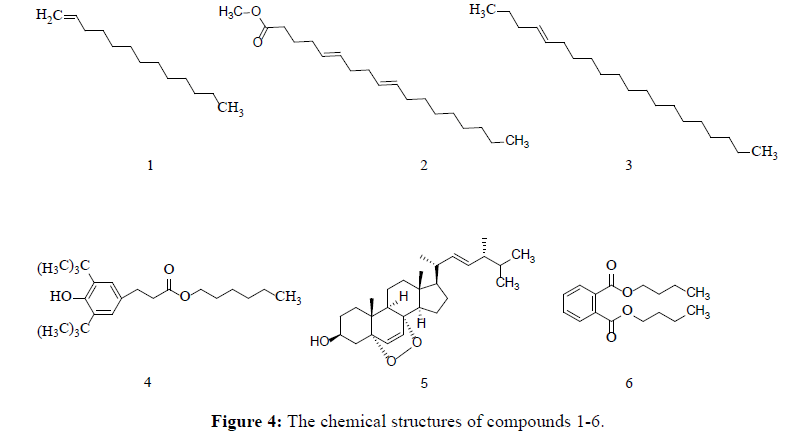
In our continuing research on metabolites of G. pannorum, we decided to further investigate the main chemical constituents responsible for fibrinolytic activities. Six small-molecular compounds (Figure 4), tridec-1-ene(1), methyl (5Z,9Z)-octadeca-5,9-dienoate(2), (E)-icos-4-ene(3), hexyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanoate(4), 5α,8α-epidioxy ergosta-6,22-diene-3β-ol (5) and dibutyl phthalate (6) were isolated and elucidated by comprehensive spectroscopic analyses including 1H NMR, 13C NMR, 1H–1HCOSY, HMBC, HMQC and MS.
Compound 1 (0.0174 g) was obtained as watery colorless liquid with a mild pleasant odor. Its molecular formula was determined to be C13H26 base on ESI-MS ([M+H] +; m/z 183), including one degree of unsaturation. The 1H NMR, 13C NMR (DEPT) data exhibited the presence of thirteen carbon resonance due to one methyne [δH 5.84–5.78 (1H, m); δC 139.2], ten methylene [δH 4.99 (1H, d, J=17.0 Hz), 4.92 (1H, d, J=10.5 Hz), δC 114.1; δH 2.04 (2H, m) δC 33.9; 1.27- 1.26 (18H, m), δC 32.0, 29.8, 29.7, 29.7, 29.6, 29.6, 29.4, 23.0 22.7], and one methyl [δH 0.88 (3H, t, J=6.5 Hz); δC 14.1], suggesting that compound 1 is long chain alkene, and the structure is determined as tridec-1-ene.
Compound 2 (0.015 g) was found to possess a molecular formula of C19H34O2 with 3 degrees of unsaturation by its ESI-MS ([M+H]+, m/z 294.3). The 1H NMR, 13C NMR (DEPT) data exhibited two double bonds [δC 130.2, 130.0, 128.0, 127.9], one carbonyl group [δC 174.2], and other twelve carbons of methylene [δC 34.1, 31.9, 29.7, 29.6, 29.4, 29.2, 27.2, 27.2, 27.1, 25.6, 24.9, 22.7], two methyl [δC 51.4, 14.1], suggesting that compound 2 is methyl (5Z,9Z)- octadeca-5,9-dienoate[22]
Compound 3 (0.0334 g) was determined to be C20H40 with one degree of unsaturation base on ESI-MS ([M+H]+, m/z 280.2). The 1H NMR, 13C NMR (DEPT) data exhibited the presence of [δH 5.34, 2.39, 2.02, 1.65, 1.25, 0.89, 0.88, 0.87] and twelve carbons [δC 130.0, 129.7, 31.9, 29.8, 29.7, 29.7, 29.7, 29.7, 29.6, 29.5, 29.5, 29.4, 29.3, 29.1, 27.2, 27.2, 22.7, 22.3, 14.2, 14.1], suggesting that compound 3 is (E)-icos-4-ene.
Compound 4 (0.0263 g), its molecular formula was deduced to be C23H38O3 base on ESI-MS ([M+H]+; m/z 263.3). The 1HNMR spectrum exhibited two aromatic hydrogen [δH 6.99 (2H, s)], seven methylene [δH 4.07 (2H, t, J=6.5 Hz), δH 2.87 (2H, t, J=7.5 Hz), δH 2.59 (2H, t, J=8.0 Hz), δH 1.25 (8H, m)], seven methy [δH 1.43 (18H, s), δH 0.88 (3H, m)]. 13CNMR spectrum exhibited 23 carbons resonance due to one carbonyl carbon [δc 173.4], six aromatic carbon [δc 152.1, 135.9, 131.2, 129.9, 129.9, 124.8], and seven methylene [64.6, 36.5, 35.9, 34.3, 27.2, 25.9, 25.6], seven methyl [31.9, 31.0, 30.3, 29.7, 29.5, 29.4, 14.1]. Compound 4 is hexyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanoate.
Compound 5 (0.078 g) was deduced to be C28H44O3 base on ESI-MS ([M+H]+, m/z 428). 13C-NMR (125 MHz, CDCl3): δC 34.7, 30.2, 66.4, 36.9, 82.2, 135.4, 130.7, 77.3, 51.1, 36.9, 23.4, 39.3, 44.6, 51.7, 20.9, 28.7, 56.2, 12.9, 18.2, 39.7, 20.6, 135.2, 132.3, 42.8, 33.1, 19.7, 19.9, 17.6. It’s identified as 5α,8α-epidioxy ergosta-6,22-diene-3β-ol in fungus Acremonium fusidioides, and its spectral data was reported in Ref. [23,24].
Compound 6 (0.0207 g) was deduced to be C16H22O4 base on ESI-MS ([M+H]+, m/z 278.1). The 1H NMR, 13C NMR spectrum exhibited a symmetry structure with one benzene ring [δH 7.71-7.72 (2H, m), δH 7.52-7.54 (2H, m), δC 132.3 (2C), 130.9 (2C), 128.9 (2C)], one carbonyl group [δC 167.7], six methylene [δH 4.29-4.32 (4H, t, J=7.5 Hz), δH 1.69- 1.75 (4H, m), δH 1.42-1.48 (4H, m), δC 65.6 (2C), 30.6 (2C), 29.7 (2C)] and two methyl [δH 0.96 (6H, t, J=7.5 Hz), δC 13.7 (2C)]. The NMR spectrum suggested that compound 6 is dibutyl phthalate.
We further demonstrated that marine-derived G. pannorum secretes large amounts of fatty chains into the culture media. The discovery of new features from marine natural products is important same as the discovery of new compounds. Marine microbes can metabolite unique compounds covering new chemical scaffold and rare substance in limited ecological environment, and the utilization of marine natural products is developing beyond its original role in identification of valuable compound leads into fields of study to new marine drug.
Fibrinolytic activities of compounds in-vitro
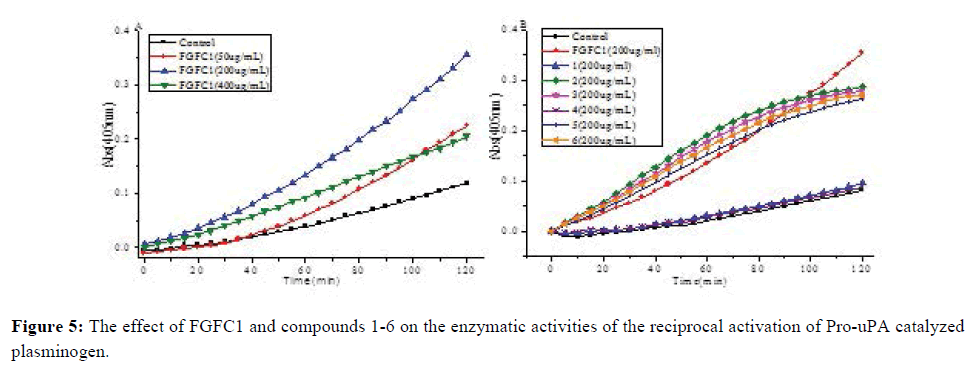
Purified compounds 1-6 were tested with concentration 50, 100, 200 and 400 μg/mL, compared to FGFC1 (EC50=113.5 μg/mL) as a reference drug (Figure 5A). Compounds 2, 3, 5, and 6 showed moderate fibrinolytic activities on concentration of 200 mg/mL in vitro with EC50 values of 120.0, 181.25, 200.0 and 179.7 μg/mL, respectively (Figure 5B). This result suggested that compounds 2, 3, 5, and 6 promote the reaction between Pro-uPA and Plg to degradation products of substitute of BSA.
Conclusion
The extracts of Geomyces sp. possessed potent antitumor, antimicrobial activity and could have a high potential as producer of antimicrobial compounds [25]. It is the first report that metabolites of G. pannorum exhibited the fibrinolytic activity. Compounds 2, 3, 5, and 6 showed moderate fibrinolytic activities in vitro with EC50 values of 120.0, 181.3, 200.0 and 179.7 μg/mL, respectively. Accordingly, the results disclosed that Geomyces sp might have abundant and cultivable microorganisms with potential metabolites for antithrombotic drugs application in biopharmaceutical industries. Marine microbes associated with polar-derived strain have received growing attention as sources of bioactive metabolites involving sustainable supplies of the marine lead compounds using biosynthesis in conjunction with synthesis and pharmacodynamic evaluation.
Acknowledgment
The work was supported by the National Natural Science Foundation of China (No. 81502955), the Open Foundation in Jiangsu Province Marine Resources Development Institute, the Young Teachers Training Program of Shanghai (No. A1-2035-15-0021-101), and the Shanghai High Technology Research and Development Program (Nos. 14431906000 and 15410722500).
References
- Elissawy AM, Ebada SS, Ashour ML, Özkaya FC, Ebrahim W, et al. (2017) Spiroarthrinols A and B, two novel meroterpenoids isolated from the sponge-derived fungus Arthrinium sp. Phytochem Lett 20: 246-251.
- Wyche TP, Standiford M, Hou Y, Braun D, Johnson DA, et al.(2013) Activation of the nuclear factor E2-related factor 2 pathway by novel natural products halomadurones A-D and a synthetic analogue. Mar Drugs 11: 5089-5099.
- Phelan RW, Barret M, Cotter PD, O'Connor PM, Chen R, et al. (2013) Subtilomycin: A new lantibiotic from Bacillus subtilis strain MMA7 isolated from the marine sponge haliclona simulans. Mar drugs 11: 1878-1898.
- Subramani R, Aalbersberg W (2013) Culturable rare Actinomycetes: diversity, isolation and marine natural product discovery. Appl Microbiol Biot 97: 9291-9321.
- Newman DJ, Cragg GM (2005) The Discovery of anticancer drugs from natural Sources. Nat Prod, (Humana Press), pp: 129-168.
- Shindo K, Misawa N (2014) New and rare carotenoids isolated from marine bacteria and their antioxidant activities. MarDrugs 12: 1690-1698.
- Newman DJ, Cragg GM (2017) Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 79: 629.
- Yang MH, Kim J, Khan IA, Walker LA, Khan SI, et al. (2014) Nonsteroidal anti-inflammatory drug activated gene-1 (NAG-1) modulators from natural products as anti-cancer agents. Life Sci 100: 75-84.
- Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, et al. (2011) 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guideli. J Am Coll Cardiol 57: 101-198.
- Su TW, Wu WH, Yan T, Zhang C, Zhu QG, et al. (2013) Pharmacokinetics and tissue distribution of a novel marine fibrinolytic compound in Wistar rat following intravenous administrations. J Chromatogr B 942: 77-82.
- Wang G, Wu WH, Zhu QG, Fu SQ, Wang XY, et al. (2015) Identification and fibrinolytic evaluation of an isoindolone derivative isolated from a rare marine fungus Stachybotrys longispora FG216. Chinese J Chem 33: 1089-1095.
- Wu WH, Hasumi K, Peng H, Hu X, Wang XY, et al.(2009) Fibrinolytic compounds isolated from a brown alga, Sargassumfulvellum. Mar Drugs 7: 85-94.
- Guo RH, Zhang YT, Duan D, Fu Q, Zhang XY, et al. (2016) Fibrinolytic evaluation of compounds isolated from a marine fungus Stachybotrys longispora FG216. Chinese J Chem 34: 1194-1198.
- Gargas A, Trest MT, Christensen M, Volk TJ, Blehert DS (2009) Geomyces destructans sp. nov. associated with bat whitenose syndrome. Mycotaxon 108: 147-154.
- Minnis AM, Lindner DL (2013) Phylogenetic evaluation of Geomyces and allies reveals no close relatives of Pseudogymnoascusdestructans, comb. nov., in bat hibernacula of eastern North America. Fungal Biol 117: 638-649.
- Li Y, Sun BD, Liu SC, Jiang L, Liu X, et al. (2008) Bioactive asterric acid derivatives from the Antarctic ascomycete fungus Geomyces sp. J Nat Prod 71: 1643-1646.
- Florczak T, Daroch M, Wilkinson MC, Bates AD, Turkiewicz M, et al. (2013) Purification, characterisation and expression in Saccharomyces cerevisiae of LipG7 an enantioselective, cold-adapted lipase from the Antarctic filamentous fungus Geomycessp. P7 with unusual thermostability characteristics. Enzyme Microb Tech 53: 18-24.
- Parish CA, de la Cruz M, Smith SK, Zink D, Baxter J, et al. (2009) Antisense-guided isolation and structure elucidation of Pannomycin, a substituted cis-decalin from Geomyces pannorum. J Nat Prod 72: 59-62.
- Astrup T, Mullertz S (1952) The fibrin plate method for estimating fibrinolytic activity [J]. Arch Biochem Biophys 40: 346.
- Sanchez JF, Somoza AD, Keller NP, Wang CC (2012) Advances in Aspergillus secondary metabolite research in the postgenomic era. Nat Prod Rep 29: 351.
- Sun X, Zhou X, Cai M, Tao K, Zhang Y (2009) Identified biosynthetic pathway of aspergiolide A and a novel strategy to increase its production in a marine-derived fungus Aspergillus glaucus by feeding of biosynthetic precursors and inhibitors simultaneously. Bioresource Technol 100: 4244-4251.
- Bus J, Sies I, Ms LKJ (1977) 13C-NMR of double and triple bond carbon atoms of unsaturated fatty acid methyl esters. ChemPhys Lipids 18: 130.
- Xiao AN, Feng BM, Chen G, Chen SF, Wang HF, et al. (2016) Isolation and identification of two new compounds from marine-derived fungus Acremonium fusidioides RZ01. Chin J Nat Med 14: 934.
- Lindequist U, Teuscher E, Wolf B, Völsgen A, Hoffmann S, et al. (1989) Isolation, characterization and structure elucidation of a substance with immunosuppressive action from Tricholoma populinum Lange. Pharmazie 44: 165.
- Henríquez M, Vergara K, Norambuena J, Beiza A, Maza F, et al. (2014) Diversity of cultivable fungi associated with Antarctic marine sponges and screening for their antimicrobial, antitumoral and antioxidant potential [J]. World J Mocrob Biot 30: 65-76.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences