ISSN : 2249 - 7412
Asian Journal of Plant Science & Research
Strategies for Secondary Metabolite Production A Review
Monisha K1*, Riddhi M1 and Parth P2
Department of Science, Navrachana University, Gujarat, India
- *Corresponding Author:
- Monisha K
Department of Science, Navrachana University, Gujarat, India
E-mail: monishak@nuv.ac.in
Received Date: April 04, 2022, Manuscript No. AJPSKY-22-12416; Editor assigned date: April 06, 2022, Pre QC No. AJPSKY-22-12416 (PQ); Reviewed date: April 15, 2022, QC No. AJPSKY-22-12416; Revised date: April 25, 2022, Manuscript No. AJPSKY-22-12416 (R); Published Date: May 02, 2022, DOI: 10.36648/2249-7412/12.5.219
Citation: Monisha K, Riddhi M, Parth P (2022) Strategies for Secondary Metabolite Production: A Review. Asian J Plant Sci Res Vol:12 No:5
Abstract
This review paper has the details on the production of secondary metabolites through plant tissue culture. It also holds information on the techniques of Plant tissue culture. Biotransformation, the conversion of less useful secondary metabolites into highly used chemical compounds is a very promising technique used to produce secondary metabolites.
Keywords
Plant tissue culture; Biotransformation; Secondary Metabolites; Agar and Agitated Cell Cultures; GC-MS; HPLC
Abbreviations
ABA: Abscise Acid; BA: Benzyl adenine; NAA: Naphthalene Acetic acid; 2,4-D: 2,4-Dichloroxyphenoxyacetic-acid; IAA: Indole-3-acetic acid; 2-iP: 2-Isopentenyladenine; LS: Linsmaier and Skoog Medium; MS: Murashige and Skoog Medium; GC-MS: Gas Chromatography-Mass spectroscopy; HPLC: High Performance Liquid Chromatography
Introduction
Plant tissue culture
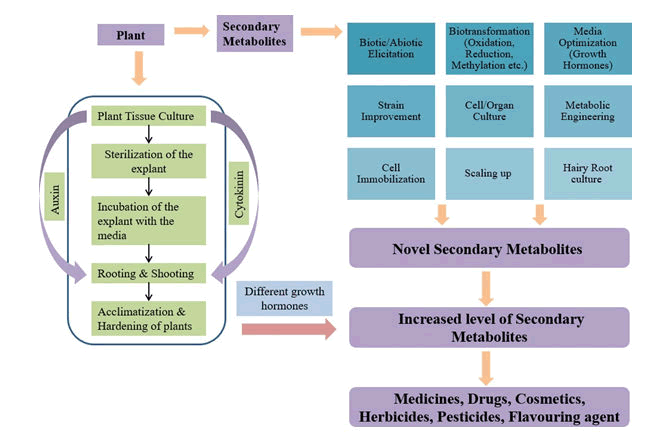
The growth and multiplication of plant’s cells, tissues and organs under controlled environment and aseptic conditions is known as Plant Tissue Culture. The establishment of this culture system has been done by Haberlandt and thereafter, it has been used by various researchers in various plant sciences field. Any part of the plant can be used for the purpose of plant tissue culture known as explants, can be grown in solid or semi-solid or liquid medium. Mostly all the parts of the plants have been used as explants in tissue culturing but axillary buds, stem-tip and meristem-tips are highly used cultures due to their own advantages [1]. The explants taken are to be surface sterilized thoroughly and then they are inoculated on a suitable medium to initiate the culturing process. The basal medium used for majority of the experiments is MS Medium and then the optimization is done according to the mode of the study [2]. The tissue culture MS Basal Medium supplies all the nutrients required for the proper growth of the plant and the stringent light and temperature conditions in the growth room adds an advantage to the entire process. Sometimes, addition of Plant growth Hormones also aids in Plant. Development Once, the mass of cells have grown or divided subsequent sub-culturing is to be done to avoid the accumulation of toxins and replacing the replenished nutrients [3-5].
Micro propagation is another word for plant tissue culture. Micropropagation is the miniaturization process of the clonal propagation [6,7]. Clonal propagation is the asexual process of producing multiple and identical copies of the plant. The process of Micropropagation is divided into five stages. The first step includes selection of the proper explant and the explant source, which should be less contamination prone [8-10].
The second step includes proper and thorough sterilization of the selected explant. The third stage includes incubation of the sterilized explant into the optimized basal medium. The fourth stage is shooting and rooting of the plant. And the last step is the most crucial and critical stage which is the acclimatization of the plant and hardening of the plant in greenhouse [9-12].
Phytohormones are the hormones in plants with the help of which plants respond to their environment. The main response of the plant takes place through the five plant hormones: Auxin, Cytokinins, Gibberellins, Abscisic Acid and Ethylene [13]. Auxins help in the growth of the plant and they are mostly present on the tip of the plant [14-18].
Cytokinins moreover help in the division of the cells and also prevent ageing. Gibberellins also help in the growth of the plant especially the stem area and it also helps to germinate the buds. Abscisic Acid prevents water loss and put the plant in dormant stage when the conditions are harsh. Ethylene helps in fruit ripening and it also has a role in abscission which is the dropping of fruits, flowers and leaves [19-22]. Auxin aids in rooting while Cytokine aids in shooting process. The ratio of the Auxin Cytokinin is particularly important. High amount of Axim leads to root development while high amount of Cytokinin leads to shoot development. Equal amount of Auxin and Cytokinin leads to callus development, which is just undifferentiated divided mass of cells. If the roots are developed first, the shoots would not develop then. So, the induction and proliferation of the shoots should be done prior to the induction of the roots [23,24].
Plant tissue culture has several applications such as in horticulture, agriculture, improvement of crops, germplasm conservation, study of plant diseases, culture of endangered plants and their preservation. The success of plant tissue culture depends on many factors such as age of the plant, genotype of the plant, the explant type and the position of the explant on the plant since the ability of expressing totipotency is different in different plants [25-28]. Since, the plants have been over-exploited and some there is a threat to some rare species so this way plant tissue culture helps in replenishing those plants [29,30]
Secondary metabolites
Secondary Metabolites are the components of the plant which are not directly involved in the growth and development of the plant like the primary metabolites [31-34]. There are many types of secondary metabolites in the plant such as alkaloids, terpenes, phenolics, flavonoids, coumarins and stilbenes, tannins, saponins and many more. They have a wide variety of applications such as pharmaceuticals, food additives, drugs and dyes, fragrances, neutraceuticals [35-37]. The amount of secondary metabolites accumulated varies in different species such as Aegle marmelos, Bletilla striata, Cassia acutifolia,Polygonum multiflorum, Zingiber officinale, Moringaoleifera, Ginkgo biloba, Eleutherococcus senticosus and other species [38-41].
This technology of isolating or production of secondary metabolites from higher plants has several advantages:
- It is a simple and straight process
- The isolation process is very efficient
- The compounds isolated work parallel with that of the natural compounds present in plants [42-48].
Plant tissue culture and secondary metabolites
The traditional methods of plant extraction and chemical synthesis of producing secondary metabolites has many flaws which can be improved through the technique of plant tissue culture. Plant tissue culture has been used for production of secondary metabolites has been going on since a long time as described [49-56].
The use of this technology is high since it provides several advantages:
- Controlled laboratory setup
- Less contamination prone if one works carefully
- Closed place where no climatic conditions and soil factors interfere
- Automatic cell growth and metabolite regulation.
The production of secondary metabolites in vitro is a two-step process:
- Biomass production and
- Secondary metabolite production
Strategies for producing secondary metabolites
Classical/conventional methods
Media optimization
The media optimization can be done through removal of some sources, supplementation of some sources or replacement of some sources. Either of these things can be done and standardized according to the feasibility of the experiment. The media can be supplemented with any of these sources such as carbon, nitrogen and amino acids or any of these in varying concentrations. The third variant used is by replacing one element with another in different combinations and checking the results. Different salts can also be added and this way the media can be improved by different techniques [57-59].
Culture condition optimization
The media can be made in duplicates and the culture conditions such as pH, temperature, incubation periods, dark and light conditions and effect of different chemicals on the media can be checked. Three to four different pH conditions and temperature variants can be checked. Different light and dark photoperiod conditions can be considered and incubation can be set accordingly [60-62].
Strain improvement
Strain improvement is done mostly to improve the metabolic engineering of the strain for biotechnological approaches. It can be done through simple genetics such as exposure to UV light or exposure to some chemical mutagens and then the screening of the improved strain is checked based on the desired genotype [63]. Other technique is advanced molecular genetics where the various biosynthetic pathways can be studied. Various vectors and genes are studied and accordingly transformation studies are done. Gene cloning and analysis can be done by this method and again screening of the strain with improved traits and desired phenotype is selected [64-68].
Somatic embryogenesis
The explants are selected from the plant of interest and is sterilized and kept in growth on MS medium under proper and suitable conditions. Callus culturing is to be done from the explants which will be obtained after 4 weeks approximately. Now, from the callus culture direct secondary metabolite production can be checked or further sub-culturing can be done for 15 days (about 2 weeks) and kept under supervision for somatic embryogenesis after addition of NAA and Kinetin. Then, histology studies can be done for the same and the embryo sections can be checked under electron microscope for the growth check [69-71].
Plant cell cultures
The plant of interest is selected. Then, they are screened for producing secondary metabolites and the best genotype is selected. The genotype is to be stabilized for efficient secondary metabolite production. Next thing is to set up the culture medium for the growth of callus which is to be prompt sub cultured and then the stable genetic line from those callus cultures is selected. Once the genetic stability is achieved further screening of the cell lines is done to produce secondary metabolites. Further from these cell lines various suspension culture studies can be done such as biosynthetic pathways, elicitation studies, immobilization and genetic modifications. Commercial production of these metabolites can also be done with the help of bioreactor systems [72-83].
Organ cell cultures
Organ cell cultures can be done in two ways: Hairy root cultures and Shoot cultures.
Hairy root cultures
The plant of interest is selected and in vitro culturing is done. The infection of the in vitro cultured roots is done with Agrobacterium rhizogenes. Incubation is done and checked for the growth of hairy roots. Hairy roots are isolated and then cultured in solid and suspension medium and plants are regenerated from the same. Further, scale up studies can be done through bioreactors [84,85].
Shoot cultures
Shoots can be developed from any explant or from the callus of the plant. The ratio of auxin to cytokinin is important here. If the focus is on the shoot growth, then the amount of cytokinin should be higher than the auxin. Once, the shoots are grown further elicitation studies can be done to check the increase in the production of secondary metabolites [86,87].
Contemporary/advanced methods
Biotic and abiotic elicitation
Biotic and Abiotic elicitors are another method used for increasing the production of secondary metabolites. Biotic elicitors include carbohydrates, proteins, plant growth promoting bacteria, different fungi and hormones. Abiotic elicitors include Heavy metals, Light, temperature, drought and salinity. Different concentration of chemicals and different combinations will have varying effects on the production of secondary metabolites. In this way, the effect of stress can be checked with these elicitors. Also, detailed biosynthetic pathway of the stress induction in plants can also be checked [88-90]
Metabolic engineering
The genetic or metabolic engineering involves the study of biosynthetic pathways, enzymatic reactions and enzymes involved in them. It also includes the modification studies in the same. The gene expression can be checked at the proteome and transcriptome level. The gene promoters and transcription factors involved in the production of secondary metabolites can also be studied. It is a particularly important technique used for scaling up the production of secondary metabolites in plants [91,92].
Role of endophytes
Entophytes are the organisms which live a considerable time of their lifespan by colonizing the living tissues of their host. Entophytic fungi are known to have symbiotic relation with some plants. During abiotic stresses or any pathogenic attack, these endophytes are known to help plants to produce secondary metabolites in defense by creating oxidative stress [93].
Plant cell immobilization and scaling up technique
The plant cell cultures from the plant of interest are immobilized in calcium alginate and the culturing of the former is done in Murashige and Skoog basal medium with different concentrations of calcium chloride to produce secondary metabolite. The secondary metabolite produced by this is compared with that of the endogenous level of secondary metabolite. Reports show that it isan important and valuable technique for scaling up the production of secondary metabolites. This also helps in the study of plant-entophytic symbiotic relationship [94-97].
Microbial biotransformation
The microbial biotransformation is carried in the following steps.
- Preparation of seed culture slants: The lyophilized culture of the strain is revived by adding it in nutrient broth and keeping it in shaking conditions at room temperature. Through serial dilutions various cultures are prepared and then streaked on petri plates and then the colonies which grow are used to prepare slants.
- Preparation of seed culture flaks: The seed flasks are prepared by picking up the colony from the slants and again kept in the shaking condition under suitable conditions.
- Strain activity test: The culture from the seed flask is taken and mixed with nutrient broth and kept in shaking conditions. The next day the sample from the flask is taken and checked under microscopy for purity.
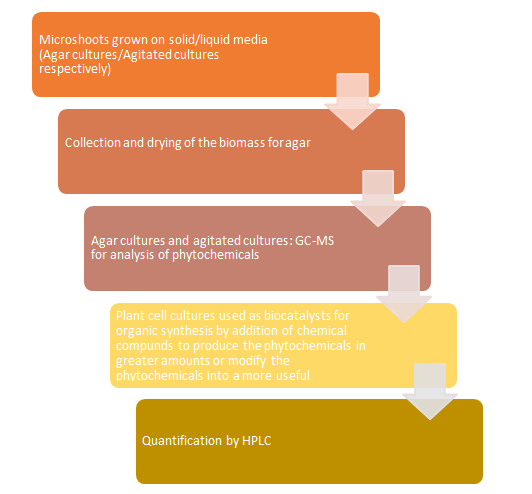
- Biotransformation: If the culture is pure then it is further kept for incubation in shaking condition for two days after adding the substrate. Then, the biotransformation is checked through HPLC or TLC (Table 1) Figure 1.
| S.no. | Plant name | Secondary metabolite | Medium | Reference |
|---|---|---|---|---|
| 1 | Agave amanuensis | Saponin | MS+Kinetin+2,4-D | Andrijany |
| 2 | Hylotelephium tatarinowii (Maxim.) | Flavonoid | MS+6-BAP+NAA+2,4-D | Wang |
| 3 | C. auranctium | Orange Flavonoids | MS+IAA+Kinetin | Brunet and Ibrahim |
| 4 | Saussurea medusa | Flavonoids | MS+NAA+6-BA | Gao |
| 5 | Ginkgo biloba | Flavonoids and Terpene Lactones | MS+NAA+6-BA | Cheng |
| 6 | Aegle marmelos | Kaempferol | MS+GrowthHormones | Talreja |
| 7 | Moringa oleifera | Quercetin | MS+GrowthHormones | Talreja |
| 8 | Zingiber officinale | Phenolic acid and Flavonoid | MS+Zeatin+NAA | Zahid |
| 9 | Polygonum multiflorum | Jasmonic Acid | MS | Ho |
| 10 | Scutellaria lateriflora | Flavones | Hormone-free MS | Barska |
| 11 | Bletilla striata | p-hydroxybenzyl alcohol | MS+6-BA+2,4-D | Pan |
| 12 | Hypercium perforatum L. | Phenolics and Flavonoids | MS+IAA | Cui |
| 13 | Ammi majus L. | Scopoletin | MS+NAA+BAP | Staniszewska |
| 14 | Rhodiola imbricateEdgew. | Phenolics and Flavonoids | MS+NAA+BAP | Kapoor |
| 15 | Artemisia annua L. | Salicylic Acid and Phenolics | MS (Hormone-free) | Kumari |
| 16 | Fagonia indica | Phenolics and Flavonoids | MS+TDZ | Khan |
| 17 | Stevia rebaudiana | Steviol glycoside | MS | Ghaheri |
| 18 | Polygonum multiflorum | Phenolic and Flavonoid | MS+IBA | Ho |
| 19 | Echium vulgare L. | Phenolic and Flavanoid | MS | Dresler |
| 20 | Phoenix dactylifera L. | Kaempferol | MS+NAA+2,4-D,2 iP | Naik and Al-Khayri |
| 21 | Dendrobium fimbriatum | Phenolic, Alkaloid, Tanninand Flavonoid | MS+BAP | Paul and Kumaria |
| 22 | Azadirachta indica | Mevalonic acid, squalene andazadirachtin | MS+Picloram+Kinetin | Farjaminezhad andGaroosi |
| 23 | Withaniasomnifera L. | Withanolide A | Hormone-free MS | Nagella andMurthy |
| 24 | Lallemantia iberica | Flavonoids and Phenolics | MS+TDZ+NAA | Pourebad |
| 25 | Artemisia annua L. | Artemisinin | MS (Hormone-free) | Wang |
| 26 | Cnidium officinale | Phthalide and 3-butylidenephthalide | MS+2,4-D+BA | Adil |
| 27 | Eleutherococcussenticosus | Eleutherosides andChlorogenic acid | MS+2,4-D | Shohaelet |
| 28 | Tripterygium wilfordii | Triptolide, Wilforgine andWilforine | MS+2,4-D+Kinetin | Miao |
| 29 | Zataria multiflora | Phenolics and Flavonoids | MS+Cytokinin+Auxin | Mosavat |
| 30 | Hyperciumhirsutum andHypercium maculatum | Hypericin and Hyperforin | MS+NAA+Kinetin | Coste |
| 31 | Lavandula spp. | Monoterpenes andSesquiterpenes | MS+Auxin+Cytokinin | Goncalves and Romano |
| 32 | Polygonum minus | Essential oils: decanal anddodecanal | MS+NAA+2,4-D | Shukor |
| 33 | Mentha pulegium | Pulegone and Menthol | MS+2,4-D | Darvishi |
| 34 | Eurycoma longifolia | Beta-carboline and Canthin-6-one | MS+2,4-D | Natanael |
| 35 | Salvia miltiorrhiza | Tanshinone | MS+2,4-D, 6-BA | Zhao |
| 36 | Ruta graveolens | Phenols and Flavonoids | MS+NAA | Sharifi |
| 37 | Corylus avellana L. | Taxol | MS+6-BA | Rezaei |
| 38 | Silybum marianumtissue | Silymarin | MS+2-IPA | AbouZid |
| 39 | Catharanthus roseus | Indole Alkaloids | MS+2,4-D+NAA+Kinetin | Zhao |
| 40 | Capsicum annuum L. | Capsaicinoids and Lignin | MS+6-BA+2,4-D | Palenius and Alejo |
| 41 | Coffea arabica | Caffeine | MS+2,4-D | Waller |
| 42 | Eriobotrya japonica | Triterpenes | LS+NAA+6-BA | Taniguchi |
| 43 | Cornuskousa | Polyphenol | MS+NAA+BA | Ishimaru |
| 44 | Eucommia ulmoides | Chlorogenic acid | MS+2,4-D+6-BA+Kinetin | Wang |
| 45 | Rubia peregrine | Anthroquinone | MS+NAA+BA | Lodhi andCharlwood |
| 46 | Chaenomeles japonica | Pentacyclic triterpenoids and Polyphenols | MS+IAA+BA | Kikowska |
| 47 | Nothapodytesnimmoniana | Camptothecin | MS+IAA+BAP | Isah and Mujib |
| 48 | Ailanthus altissima | Alkaloid | MS+2,4-D+Kinetin | Anderson |
| 49 | Cassia acutifolia | Anthroquinone | MS+2,4-D+Kinetin | Nazif |
| 50 | Rheum franzenbachii | Rhaponticin | MS+6-BAP+NAA | Wang |
| 51 | Camptotheca acuminate | Camptothecin and 10-hydroxycamptothecin | MS+NAA+Kinetin | Wiedenfeld |
| 52 | Angelica dahurica | Imperatorin | MS+BA | Tsay |
| 53 | Citrus foliage | Glycoproteins | MS+NAA+ABA | Witt |
| 54 | Cinchona ledgeriana | Alkaloid | MS+2,4-D+Kinetin | Anderson |
| 55 | Stephania cepharantha | Biscoclaurine | MS+2,4-D+IAA | Akasu |
Table 1: Secondary metabolites produced by plant tissue culture in different plant species.
Biotransformation
Biotransformation of secondary metabolites is a process in plant which can be defined as conversion of the chemical compound into some new chemical compound which is of potential importance by biological means. Methylation, Oxidation, Esterification, Reduction, Glycosylation, Isomerization and [98-105].
Hydroxylation are some of the reactions through which biotransformation can be carried out. There is an alternative for the production of secondary metabolites and that is the production of secondary metabolites through plant cell cultures. Hydroxylation is possible in compounds which have oxygenated positions in it and can be exploited for conversion into some valuable products. Oxidation and Reduction can also take in some selected group of plants and is an important reaction of the biotransformation process where the hydroxyl groups are oxidated [106,107].
The alcohol is converted to ketones by the plant cell cultures and the corresponding aldehydes and ketones can also get converted to alcohols back by the plant cell cultures. Glycosylation is another reaction which occurs in plant cell cultures during low substrate concentration. It also takes place during toxicity by converting one compound to another less toxic compound and thereby helps in medicine sector. In a layman’s language Esterification is the reaction where at least one ester group is formed when two compounds react. If the compound has one hydroxyl group on the steroid, then it can react and form a conjugate with the fatty acid and as a result esterification can take place. Acetylation is the reaction where the hydrogen atom is substituted or replace by the acetyl group and the same mechanism applies during biotransformation too [108-110]. The reaction has been known to have many applications in producing useful secondary metabolites. Isomerization, another reaction for biotransformation is an incomplete reaction which leads to the formation of different substrates and products due to thermodynamic equilibrium. The amount of product accumulated by different enzymatic reactions depends on the capacity of the cells, variation and the stability of the cells. Numerous factors affecting the process of biotransformation are Media composition especially Carbon, Nitrogen and Phosphorus, Growth Regulators and Light [110-117].
The Table 2 given below depicts the secondary metabolites produced by various plant species through plant cell cultures by Biotransformation Figure 2.
| S. No. | Plant species | Secondary metabolite | Reference |
|---|---|---|---|
| 1 | Atropa belladonna | Hyoscyamine and Scopolamine | Subroto |
| 2 | Duboisia spp. | Hyoscyamine | Subroto |
| 3 | Eucalyptus | Beta-Thujaplicin | Furuya |
| 4 | Scutellaria lateriflora L. | Flavonoids and Verbascoside | KawkaB |
| 5 | Hypercium perforatum | Phenolic Acid | Kwiecien |
| 6 | Scutellaria baicalensis | Flavonoids and Verbascoside | Kawka |
| 7 | Aronia arbutifolia | Hydroquinone and Arbutin | Szopa |
| 8 | Cistus x incanus L. | Gallic acid, Catechins andFlavonoids | Kubica |
| 9 | Eryngium alpinum L. | Phenolic Acid and Flavonoids | Kikowska |
| 10 | Cistus x incanus | Phenolic Acids, Catechins and Flavonoids | Dziurka |
| 11 | Verbena officinalis | Phenolic Acids and Phenylethanoid glycosides | Dziurka |
| 12 | Scutellaria baicalensis | Phenolic Acids, Flavonoids and Phenylethanoid glycosides | Dziurka |
| 13 | Scutellaria lateriflora | Phenolic Acids, Flavonoids and Phenylethanoid glycosides | Dziurka |
| 14 | Schisandra chinensis (Turcz.) | Phenolic Acids and Flavonoids | Szopa |
| 15 | Schisandra chinensis (Turcz.) | Dibenzocyclooctadiene lignans | Szopa |
| 16 | Schisandra chinensis cv. | Phenolic Acid and Flavonoid | Szopa |
| 17 | Capsicum frutescens | Phenyl propanoids | Suresh and Ravishankar |
| 18 | Origanum majorana L. | Hydroquinone | Pietraszek |
| 19 | Lavandula augustifolia | Monoterpenoids | Lappin |
| 20 | Micrococcus luteus | Oleic Acid | Boratynski |
| 21 | Bacillus safensisSMS1003 | Eugenol | Singh |
| 22 | Melittis melissophyllum L. | Harpagide and 8-O-acetyl harpagide | Pietraszek |
| 23 | Pseudomonas aeruginosa | Melanin | Bolognese |
| 24 | Leptosphaerulina sp. | Cephadroxyl | Perez-Grisales |
| 25 | Schisandra chinensis | Hydroquinone and 4-hydroxybenzoic acid | Szopa |
Table 2: Biotransformation of secondary metabolites in different plant species.
Conclusion
There are numerous numbers of methods which can be used for producing secondary metabolites and the elicitation for the same. The appropriate method to be used depends on the plants and their characteristics.
Different methods have different effect on the plants and accordingly the best technique suitable for the plants can be manipulated, combined and applied. The choice of technique used for enhancement of these metabolites needs to be explored in more plants to understand its efficacy in a better way.
Statements and Declarations
Funding
“No grants, funds or any kind of financial aid were taken while preparing this manuscript”
Competing Interests
“There are no financial or non-financial interests to show or disclose by any of the authors”
Author Contribution
“All the authors have contributed in the work and have gone through the final work. All the authors take equal responsibility for the integrity and publication of the work.”
References
- Vasil IK, Vasil V (1972) Totipotency and embryogenesis in plant cell and tissue cultures. In vitro 8:117-127
- Gamborg OL, Murashige T, Thorpe TA, Vasil IK (1976) Plant tissue culture media. In vitro 12:473-478
- Bhojwani SS, Dantu PK (2013) Micro propagation. In Plant tissue culture: An introductory text, Springer, India. Pp245-274
- Puhan Z, Martin SM (1971) The industrial potential of plant cell culture. Progress in industrial microbiology 9:13-39
- Chandana BC, Kumari Nagaveni HC, Heena MS, Shashikala SK, Lakshmana D, et al. (2018) Role of plant tissue culture in micropropagation, secondary metabolites production and conservation of some endangered medicinal crops. J Pharmacogn Phytochem 3:246-251
- Ramesha BT, Amna T, Ravikanth G, Gunaga RP, Vasudeva R, et al. (2008) Prospecting for camptothecines from Nothapodytes nimmoniana in the Western Ghats, South India: identification of high-yielding sources of camptothecin and new families of camptothecines. J Chromatogr Sci 46:362-368
- Hussain MS, Fareed S, Saba Ansari M, Rahman A, et al. (2012) Current approaches toward production of secondary plant metabolites. J Pharm Bioallied Sci 4:10-20
- Kolewe ME, Gaurav V, Roberts SC (2008) Pharmaceutically active natural product synthesis and supply via plant cell culture technology. Mol Pharm 5:243-256
- Murthy HN, Lee EJ, Paek KY (2014) Production of secondary metabolites from cell and organ cultures: strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Organ Cult 118:1-16
- Singh V, Haque S, Niwas R, Srivastava A, Pasupuleti M, et al. (2017) Strategies for fermentation medium optimization: an in-depth review. Front Microbiol 7:2087
- Khattab AI, Babiker EH, Saeed HA (2016) Streptomyces: isolation, optimization of culture conditions and extraction of secondary metabolites. Int curr pharm J 5:27-32
- Gonzalez JB, Fernandez FJ, Tomasini A (2003) Microbial secondary metabolites production and strain improvement. India J Biotechnol 2:322-333
- Mathur J (1993) Somatic embryogenesis from callus cultures of Nardostachys jatamansi. Plant Cell Tissue Organ Cult 33:163-16
- Bourgaud F, Gravot A, Milesi S, Gontier E (2001) Production of plant secondary metabolites:a historical perspective. Plan scie 161:839-851
- Pistelli L, Giovannini A, Ruffoni B, Bertoli A, Pistelli L, et al. (2010) Hairy root cultures for secondary metabolites production. Adv Exp Med Biol 698:167-184
- Naik PM, Al-Khayri JM (2016) Abiotic and biotic elicitors–role in secondary metabolites production through in vitro culture of medicinal plants. Intech Open, India. Pp 247-277
- Thakur M, Bhattacharya S, KhoslaPK, PuriS (2019) Improving production of plant secondary metabolites through biotic and abiotic elicitation. J Appl Res Med Aromat Plants 12:1-12
- Gonçalves, Sandra, Romano, Anabela (2018) Production of Plant Secondary Metabolites by Using Biotechnological Tools. Intech open, India.
[Crossref]
- Benedito, Vagner, Modolo, Luzia (2013) Introduction to Metabolic Genetic Engineering for the Production of Valuable Secondary Metabolites in in vivo and in vitro Plant Systems. Recent Pat Biotechnol 8:61-75
- Zhang HW, Song YC, Tan RX (2006) Biology and chemistry of endophytes. Nat Prod Rep 23:753-771
- Ogbe AA, Finnie JF, Van Staden J (2020) The role of endophytes in secondary metabolites accumulation in medicinal plants under abiotic stress. S Afr J Bot 134:126-134
- Prakash S, Bajaj A (2017) Process for biotransformation of androsta-4-ene-3, 17-dione (4-AD) to androsta-1, 4-diene-3, 17-dione (ADD). Method Mol Biol Humana Press NewYork 1645:227-238
- Furuya T, Asada Y, Matsuura Y, Mizobata S, Hamada H, et al. (1997) Biotransformation of ß-thujaplicin by cultured cells of Eucalyptus perriniana. Phytochemistry 46:1355-1358
- Rao SR, Ravishankar GA (2002) Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv 20:101-153
- Suga T, Hirata T (1990) Biotransformation of exogenous substrates by plant cell cultures. Phytochem 29:2393-2406
- Nunes IS, Faria JM, Figueiredo AC, Pedro LG, Trindade H, et al. (2009) Menthol and geraniol biotransformation and glycosylation capacity of Levisticum officinale hairy roots. Planta med 75:387-391
- Schwarz TI, Katsiadaki I, Maskrey BH, Scott AP (2017) Rapid uptake, biotransformation, esterification and lack of depuration of testosterone and its metabolites by the common mussel, Mytilus spp. J Steroid Biochem Mol Biol 171:54-65
- Sun CF, Li YQ, Mao XM (2020) Regulation of protein post-translational modifications on metabolism of actinomycetes. Biomolecules 10:1122
- Liu JJ, ZhangC, Kwak S, Oh EJ, Yun EJ, et al. (2019) Overcoming the thermodynamic equilibrium of an isomerization reaction through oxidoreductive reactions for biotransformation. Nat Commun 10:1-8
- Kurz WGW, Constabel F, Alfermann AW (1984) Aspects affecting biosynthesis and biotransformation of secondary metabolites in plant cell cultures. Critical Reviews in Biotechnology 2:105-118
- AbouZid S (2014) Yield improvement strategies for the production of secondary metabolites in plant tissue culture: silymarin from Silybum marianum tissue culture. Nat Prod Res 28:2102-2110
- Adali M B, Barresi AA, Boccardo G, Pisano R (2020) Spray freeze-drying as a solution to continuous manufacturing of pharmaceutical products in bulk. Proce 8:709
- Akasu M, Itokawa H, Fujita M (1976) Biscoclaurine alkaloids in callus tissues of Stephania cepharantha. Phytochem 15:471-473
- Amini S, Maali-Amiri R, Mohammadi R, Kazemi-Shahandashti SS (2017) cDNA-AFLP analysis of transcripts induced in chickpea plants by TiO2 nanoparticles during cold stress. Plant Physiol Biochem 111:39-49
- Anderson LA, Keene AT, Phillipson JD (1982) Alkaloid production by leaf organ, root organ and cell suspension cultures of Cinchona ledgeriana. Planta Med 46:25-27
- Anderson LA, Roberts MF, Phillipson JD (1987) Studies on Ailanthus altissima cell suspension cultures. The effect of basal media on growth and alkaloid production. Plant Cell Rep 6:239-241
- Babushkina EA, Belokopytova LV, Grachev AM, Meko DM, Vaganov EA, et al. (2017) Variation of the hydrological regime of Bele-Shira closed basin in Southern Siberia and its reflection in the radial growth of Larix sibirica. Reg Environ Change 17:1725-1737
- Bhatia S, Bera T, Dahiya R, Bera T, Bhatia S, et al. (2015) Classical and nonclassical techniques for secondary metabolite production in plant cell culture. Mod Applic Plan Biotechnol Pharmac Scie 231-291
[Crossref]
- Bhojwani SS, Razdan MK (1986) Plant tissue culture: theory and practice. (1steddition), Elsevier, India.
- Bolognese F, Scanferla C, Caruso E, Orlandi VT (2019) Bacterial melanin production by heterologous expression of 4-hydroxyphenylpyruvate dioxygenase from Pseudomonas aeruginosa. Int J Biol Macromol 133:1072-1080
- Boratynski F, Szczepanska E, De Simeis D, Serra S, Brenna E, et al. (2020) Bacterial biotransformation of oleic acid: New findings on the formation of ?-dodecalactone and 10-ketostearic acid in the culture of Micrococcus Luteus. Mole 25:3024
- Brunet G, Ibrahim RK (1973) Tissue culture of Citrus peel and its potential for flavonoid synthesis. Zeitschrift für Pflanzenphysiologie 69:152-162
- Buitelaar RM, Tramper J (1992) Strategies to improve the production of secondary metabolites with plant cell cultures: a literature review. J Biotechnol 23:111-141
- Chandran H, Meena M, Barupal T, Sharma K (2020) Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol Rep 26:00450
- Cheng S, Zhang W, Nannan SUN, Feng XU, Linling LI, et al. (2014) Production of flavonoids and terpene lactones from optimized Ginkgo biloba tissue culture. Not Bot Horti Agrobot Cluj-Nap 42:88-93
- Coste A, Vlase L, Halmagyi A, Deliu C, Coldea G, et al. (2011) Effects of plant growth regulators and elicitors on production of secondary metabolites in shoot cultures of Hypericum hirsutum and Hypericum maculatum. Plant Cell Tissue Organ Cult 106:279-288
- Cui XH, Chakrabarty D, Lee EJ, Paek KY (2010) Production of adventitious roots and secondary metabolites by Hypericum perforatum L. in a bioreactor. Bioresour Technol 101:4708-4716
- Darvishi E, Kahrizi D, Bahraminejad S, Mansouri M (2016) In vitro induction of a-pinene, pulegone, menthol, menthone and limonene in cell suspension culture of pennyroyal (Mentha pulegium). Cell Mol Biol 62:7-9
- Dias MI, Sousa MJ, Alves RC, Ferreira IC (2016) Exploring plant tissue culture to improve the production of phenolic compounds: A review. Industrial crops and products, 82:9-22
- Dresler S, Rutkowska E, Bednarek W, Stanislawski G, Kubrak T, et al. (2017) Selected secondary metabolites in Echium vulgare L. populations from nonmetalliferous and metalliferous areas. Phytochem 133:4-14
- Dziurka M, Kubica P, Kwiecien I, Biesaga-Koscielniak J, Ekiert H, et al. (2021) In Vitro Cultures of Some Medicinal Plant Species (Cistus× incanus, Verbena officinalis, Scutellaria lateriflora, and Scutellaria baicalensis) as a Rich Potential Source ofAntioxidants—Evaluation by CUPRAC and QUENCHER-CUPRAC Assays. Plants 10:454
- Espinosa-Leal CA, Puente-Garza CA, García-Lara S (2018) In vitro plant tissue culture: means for production of biological active compounds. Planta 248:1-18
- Farjaminezhad R, Garoosi G (2021) Improvement and prediction of secondary metabolites production under yeast extract elicitation of Azadirachta indica cell suspension culture using responsesurface methodology. AMB Express 11:1-16
- Gao M, Song BZ, Liu CZ (2006) Dynamic microwave-assisted extraction of flavonoids from Saussurea medusa Maxim cultured cells. Biochem Eng J 32:79-83
- Ghaheri M, Kahrizi D, Bahrami G, Mohammadi-Motlagh HR (2019) Study of gene expression and steviol glycosides accumulation in Stevia rebaudiana Bertoni under various mannitol concentrations. Mol Biol Rep 46:7-16
- Gonçalves S, Romano A (2013) In vitro culture of lavenders (Lavandula spp.) and the production of secondary metabolites. Biotechnology advances, 31:166-174
- Ho TT, Lee JD, Jeong CS, Paek KY, Park SY (2018) Improvement of biosynthesis and accumulation of bioactive compounds by elicitation in adventitious root cultures of Polygonum multiflorum. Appl Microbiol Biotechnol 102:199-209
- Ho TT, Murthy HN, Park SY (2020) Methyl jasmonate induced oxidative stress and accumulation of secondary metabolites in plant cell and organ cultures. Int J Mol Sci 21:716
- Hwang SJ, Kim KS, Pyo BS, Hwang B (1999) Saponin production by hairy root cultures of Panax ginseng CA Meyer: influence of PGR and polyamines. Biotechnol Bioprocess Eng 4:309-312
- Isah T, Mujib A (2015) In vitro propagation and camptothecin production in Nothapodytes nimmoniana. Plant Cell, Tissue and Organ Cult 121:1-10
- Ishimaru K, Arakawa H, Neera S (1993) Polyphenol production in cell cultures of Cornus kousa. Phytochemistry, 32:1193-1197
- Kapoor S, Raghuvanshi R, Bhardwaj P, Sood H, Saxena S, et al. (2018) Influence of light quality on growth, secondary metabolites production and antioxidant activity in callus culture of Rhodiola imbricata Edgew. J Photochem Photobiol B 183:258-265
- Kawka B, Kwiecien I, Ekiert H (2020) Endogenous production of specific flavonoids and verbascoside in agar and agitated microshoot cultures of Scutellaria lateriflora L. and biotransformation potential. Plant Cell, Tissue and Organ Cult 142:471-482
- Kawka B, Kwiecien I, Ekiert HM (2021) Production of specific flavonoids and verbascoside in shoot cultures of Scutellaria baicalensis. Plant Cell and Tissue Differentiation and Secondary Metabolites: Fundamentals and Applications 249-272
- Khan T, Hano C, Abbasi BH (2019) Effects of chitosan and salicylic acid on the production of pharmacologically attractive secondary metabolites in callus cultures of Fagonia indica. Industrial Crops and Products 129:525-535
- Kikowska M, Thiem B, Szopa A, Ekiert H (2020) Accumulation of valuable secondary metabolites: phenolic acids and flavonoids in different in vitro systems of shoot cultures of the endangered plant species-Eryngium alpinum L. Plant Cell, Tissue and Organ Cult 141:381-391
- KikowskaM, WlodarczykA, StochmalA, Zuchowski J, ThiemB, et al. (2019) Pentacyclic triterpenoids and polyphenols accumulation in cell suspension culture of Chaenomeles japonica (Thunb.) Lindl. ex Spach. Herba Polonica 65:11
- Krikorian AD (1995) Hormones in tissue culture and micropropagation. In Plant hormones 774-796 Springer, Dordrecht.
- Kubica P, Szopa A, Ekiert H (2017) In vitro shoot cultures of pink rock-rose (Cistus x incanus L.) as a potential source of phenolic compounds. Acta Societatis Botanicorum Poloniae 86:3563
- Kumari A, Pandey N, Pandey-Rai S (2018) Exogenous salicylic acid-mediated modulation of arsenic stress tolerance with enhanced accumulation of secondary metabolites and improved size of glandular trichomes in Artemisia annua L. Protoplasm 25:139-152
- Kwiecien I, Szydlowska A, Kawka B, Beerhues L, Ekiert H, et al. (2015) Accumulation of biologically active phenolic acids in agitated shoot cultures of three Hypericum perforatum cultivars:‘Elixir’,‘Helos’ and ‘Topas’. Plant Cell Tissue and Organ Cult 123:273-281
- Le Roux JJ, Van Staden J (1991) Micropropagation and tissue culture of Eucalyptus—a review. Tree Physiol 9:435-477
- Lodhi AH, Charlwood BV (1996) Agrobacterium rhizogenes-mediated transformation ofRubia peregrina L: in vitro accumulation of anthraquinones. Plant cell, tissue and organ culture 46:103-108
- Ma WL, Yan CY, Zhu JH, Duan GY, Yu RM, et al. (2010) Biotransformation of paeonol and emodin by transgenic crown galls of Panax quinquefolium. Appl Biochem Biotechnol 160:1301-1308
- Miao GP, Zhu CS, Yang YQ, Feng MX, Ma ZQ, et al. (2014) Elicitation and in situ adsorption enhanced secondary metabolites production of Tripterygium wilfordii Hook. f. adventitious root fragment liquid cultures in shake flask and a modified bubble column bioreactor. Bioprocess Biosyst Eng 37:641-650
- Mosavat N, Golkar P, Yousefifard M, Javed R (2019) Modulation of callus growth and secondary metabolites in different Thymus species and Zataria multiflora micropropagated under ZnO nanoparticles stress. Biotechnol Appl Biochem 66:316-322
- Nagella P, Murthy HN (2011) Effects of macroelements and nitrogen source on biomass accumulation and withanolide-A production from cell suspension cultures of Withania somnifera (L.) Dunal. Plant Cell Tissue and Organ Cult 104:119-124
- Naik PM, Al-Khayri JM (2017) Extraction and estimation of secondary metabolites from date palm cell suspension cultures. Humana Press, New York, NY. pp. 319-332
- Natanael J, Esyanti RR, Manurung R (2014) Growth kinetics and secondary metabolite production of Eurycoma longifolia Jack cell culture elicitated by UV in flask scale and bubble column bioreactor scale. Int J Tech Res Appl 2:29-32
- Nazif NM, Rady MR, El-Nasr MS (2000) Stimulation of anthraquinone production in suspension cultures of Cassia acutifolia by salt stress. Fitoterapia 71:34-40
- Noreen S, Siddiq A, Hussain K, Ahmad S, Hasanuzzaman M (2017) Foliar application of salicylic acid with salinity stress on physiological and biochemical attributes of sunflower (Helianthus annuus L.) crop Acta Sci Pol Hortorum Cultus 16:57-74
- Nunez-Palenius HG, Ochoa-Alejo N (2005) Effect of phenylalanine and phenylpropanoids on the accumulation of capsaicinoids and lignin in cell cultures of chili pepper (Capsicum annuum L.). In Vitro Cell Dev Biol Plant 41:801-805
- Otles S, Özyurt VH (2021) Biotransformation in the production of secondary metabolites. Stud Nat Prod Chem 68:435-457
- Paul P, Kumaria S (2020) Precursor-induced bioaccumulation of secondary metabolites and antioxidant activity in suspension cultures of Dendrobium fimbriatum, an orchid of therapeutic importance. S Afr J Bot 135:137-143
- Pérez-Grisales MS, Castrillón-Tobón M, Copete-Pertuz LS, Plácido J, Mora-Martínez AL (2019) Biotransformation of the antibiotic agent cephadroxyl and the synthetic dye Reactive Black 5 by Leptosphaerulina sp. immobilised on Luffa (Luffa cylindrica) sponge. Biocatal Agric Biotechnol 18:101051
- Phillips GC, Garda M (2019) Plant tissue culture media and practices: an overview. In Vitro Cell Dev Biol-Plant 55:242-257
- Pourebad N, Motafakkerazad R, Kosari-Nasab M, Akhtar NF, Movafeghi A (2015) The influence of TDZ concentrations on in vitro growth and production of secondary metabolites by the shoot and callus culture of Lallemantia iberica. Plant Cell Tissue Organ Cult 122:331-339
- Rezaei A, Ghanati F, Behmanesh M, Mokhtari-Dizaji M (2011) Ultrasound-potentiated salicylic acid–induced physiological effects and production of taxol in hazelnut (Corylus avellana L.) cell culture. Ultrasound Med Biol biology 37:1938-1947
- Sharifi Y, Omran VG, Ghavami TST, Gharakhili AN, Ebrahimzadeh MA, et al. (2019) Effect of Salicylic acid on Phenols and flavonoids content and DPPH scavenging activity in cell suspension culture of Iranian sodab (Ruta graveolens). Tabari Biomed Stu Res J 1:18-21
[Crossref]
- Shohael AM, Ali MB, Yu KW, Hahn EJ, Islam R, et al. (2006) Effect of light on oxidative stress, secondary metabolites and induction of antioxidant enzymes in Eleutherococcus senticosus somatic embryos in bioreactor. Process Biochem 41:1179-1185
- Shukor MFA, Ismail I, Zainal Z, Noor NM, et al. (2013) Development of a Polygonum minus cell suspension culture system and analysis of secondary metabolites enhanced by elicitation. Acta Physiol Plant 35:1675-1689
- Singh A, Mukhopadhyay K, Ghosh Sachan S (2019) Biotransformation of eugenol to vanillin by a novel strain Bacillus safensis SMS1003. Biocatal Biotransformation 37:291-303
- Skrzypczak-Pietraszek E, Kwiecien I, Goldyn A, Pietraszek J (2017) HPLC-DAD analysis of arbutin produced from hydroquinone in a biotransformation process in Origanum majorana L. shoot culture. Phytochem Lett 20:443-448
- Skrzypczak-Pietraszek E, Reiss K, Zmudzki P, Pietraszek J (2018) Enhanced accumulation of harpagide and 8-O-acetyl-harpagide in Melittis melissophyllum L. agitated shoot cultures analyzed by UPLC-MS/MS. Plos one 13:0202556
- Smitha MS, Singh S, Singh R (2017) Microbial biotransformation: a process for chemical alterations. J Bacteriol Mycol Open Access 4:47-51
- Staniszewska I, Królicka A, Malinski E, Lojkowska E, Szafranek J (2003) Elicitation of secondary metabolites in in vitro cultures of Ammi majus L. Enzym Microb Technol 33:565-568
- Steinfeld B, Scott J, Vilander G, Marx L, Quirk M, et al. (2015) The role of lean process improvement in implementation of evidence-based practices in behavioral health care. J Behav Health Serv Res 42:504-518
- Subroto MA, Kwok KH, Hamill JD, Doran PMb (1996) Coculture of genetically transformed roots and shoots for synthesis, translocation, and biotransformation of secondary metabolites. Biotechnol Bioeng 49:481-494
- Szopa A, Dziurka M, Granica S, Klimek-Szczykutowicz M, Kubica P, et al. (2020) Schisandra rubriflora Plant Material and In Vitro Microshoot Cultures as Rich Sources of Natural Phenolic Antioxidants. Antioxidants (Basel) 9:488
- Szopa A, Klimek-Szczykutowicz M, Kokotkiewicz A, Dziurka M, Luczkiewicz M, et al. (2019) Phenolic acid and flavonoid production in agar, agitated and bioreactor-grown microshoot cultures of Schisandra chinensis cv. Sadova No. 1–a valuable medicinal plant. J Biotechnol 305:61-70
- Szopa A, Kokotkiewicz A, Bednarz M, Luczkiewicz M, Ekiert H, et al. (2017) Studies on the accumulation of phenolic acids and flavonoids in different in vitro culture systems of Schisandra chinensis (Turcz.) Baill. using a DAD-HPLC method. Phytochem Lett 20:462-469
- Szopa A, Kokotkiewicz A, Marzec-Wróblewska U, Bucinski A, Luczkiewicz M, et al. (2016) Accumulation of dibenzocyclooctadiene lignans in agar cultures and in stationary and agitated liquid cultures of Schisandra chinensis (Turcz.) Baill. Appl Microbiol Biotechnol 100:3965–3977
- Szopa A, Kubica P, Ekiert H (2018) Agitated shoot cultures of Aronia arbutifolia and Aronia×prunifolia: biotechnological studies on the accumulation of phenolic compounds and biotransformation capability. Plant Cell, Tissue and Organ Cult 134:467-479
- Taniguchi S, Imayoshi Y, Kobayashi E, Takamatsu Y, Ito H, et al. (2002) Production of bioactive triterpenes by Eriobotrya japonica calli. Phytochem 59:315-323
- Tsay HS, Chang WD, Chen CC, Chang YS (1994) The production of imperatorin from angelica dahurica var Formosana by cell suspension culture. J Agricult Asso China
- Verpoorte R, Contin A, Memelink J (2002) Biotechnology for the production of plant secondary metabolites. Phytochem Rev 1:13-25
- Waller GR, MacVean CD, Suzuki T (1983) High production of caffeine and related enzyme activities in callus cultures of Coffea arabica L. Plant Cell Rep 2:109-112
- Wang H, Ma C, Li Z, Ma L, Wang H, et al. (2010) Effects of exogenous methyl jasmonate on artemisinin biosynthesis and secondary metabolites in Artemisia annua L. Ind Crops Prod 31:214-218
- Wang J, Liao X, Zhang H, Du J, Chen P (2003) Accumulation of chlorogenic acid in cell suspension cultures of Eucommia ulmoides. Plant cell Tissue and organ cult 74:193-195
- Wang J, Lu Y, Wang Q, Liu K, Song Y, et al. (2011) An efficient callus proliferation protocol and rhaponticin accumulation of Rheum franzenbachii Munt, a medicinal plant J Plant Biochem Biotechnol 20:252-257
- Wang J, Wang J, Liu K, Xiao X, Gong W, et al. (2010) An efficient plant regeneration system with in vitro flavonoid accumulation for Hylotelephium tatarinowii (Maxim.) H. Ohba. In Vitro Cell Dev Biol Plant 46:445-450
- Wiedenfeld H, Furmanowa M, Roeder E, Guzewska J, Gustowski W (1997) Camptothecin and 10-hydroxycamptothecin in callus and plantlets of t Camptotheca acuminate. Plant Cell, Tissue and Organ Cult 49:213-218
- Witt W, Mauk CS, Yelenosky G (1989) Glycoproteins in citrus foliage and callus tissue as affected by cold-hardening temperature and abscisic acid. J Plant Physiol 134:716-721
- Xiao X, Wu ZC, Chou KC (2011) A multi-label classifier for predicting the subcellular localization of gram-negative bacterial proteins with both single and multiple sites. PloS one 6:20592
- Zahid NA, Jaafar HZ, Hakiman M (2021) Micropropagation of ginger (Zingiber officinale Roscoe)‘Bentong’and evaluation of its secondary metabolites and antioxidant activities compared with the conventionally propagated plant. Plants 10:630
- Zhao JL, Zhou LG, Wu JY (2010) Effects of biotic and abiotic elicitors on cell growth and tanshinone accumulation in Salvia miltiorrhiza cell cultures. Appl Microbiol Biotechnol 87:137-144
- Zhao J, Zhu WH, Hu Q, Guo YQ (2001) Compact callus cluster suspension cultures of Catharanthus roseus with enhanced indole alkaloid biosynthesis. In Vitro Cell Dev Biol Plant 37:68-72

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences