ISSN : 0976-8505
Der Chemica Sinica
Stereo Chemistry Explain the Activity Toxicity and the Pharmacological Action of Many Drugs
Rezk Ayad*
Department of Pharmaceutical Chemistry, Al-Azhar University, Cairo, Egypt
- *Corresponding Author:
- Rezk Ayad
Department of Pharmaceutical Chemistry, Al-Azhar University, Cairo, Egypt
Tel: 01225432266
E-mail: rezek_ayad@yahoo.com
Received Date:March 23, 2022, Manuscript No. IPDCS-22-12301; Editor assigned date: March 25, 2022, PreQC No. IPDCS-22-12301 (PQ); Reviewed date: April 08, 2022, QC No. IPDCS-22-12301; Revised date: May 23, 2022, Manuscript No. IPDCS-22-12301 (R); Published date: June 01, 2022, DOI: 10.36648/0976-8505/13.6.001
Citation: Rezk A (2022) Stereo Chemistry Explain the Activity, Toxicity and the Pharmacological Action of Many Drugs. Der Chem Sin Vol:13 No:6
Abstract
Many drugs differ in pharmacological action according to their stereochemistry. The quinine used as antimalarial while quinidine used as antiarrhythmic, dextro cyclophosphamide is toxic while the levo is anticancer, dextro ketamine used as general anathesia while levo ketamine is agitation agent, dextro secobarbital is anticonvulsant while levo secobarbital is anesthetic, levodopa is antiparkinsonism while dopa is a granulocytosis, levamisole is safe anthelmintic while tetramisole has many side effects and less active, Thalidomide the (R) form more safe than (S) form which is tratogenic, the cisplatin is more active than the trans form, the levo adrenaline is more active than dextro form the diethylstilbosterol the transform is more active than cis form.
Keywords
Stereochemistry; Levo; Dextro; Cis; Trans; Active; Inactive
Introduction
In 1948, Luis pastier reveals the expression of stereochemistry and explains what is chiral carbon when he presents the tartaric acid in two forms where the two forms differ in their crystals. Nearly most of the drugs which used in medicine are having chiral carbon(s) [1-5]. All natural amino acids are levo form and all natural sugars are dextro form. The expression of stereo isomer mean enantiomer means disassymetry. The Chirality means handedness also the chiral carbon equal stereo genic carbon which mean chiral center. (I.e. the chiral center may be sulfur, phosphorus or nitrogen instead of carbon). Enantiomer mean optical isomer which either levo or dextro [6-9]. The (R) form (Rectus) mean deviate the polarized light with clockwise while the (S) form sinister mean deviate the polarized light against clockwise (anti clockwise) [10]. Diastereomers mean the molecule has to chiral carbon. Eutomer main bioactive enantiomer i.e. having higher biological activity. Enantio selectivity means enzymes antibodies or receptors having selective bind to the compounds (used in enzyme assay) [11-18]. Homochirality mean there are many compounds having the same chiral carbon e.g. amino acids. Chiral switch mean racemic. The eutomer is versus diastomer where the eutomer is highly biological active while the diastomer is less active. Many drugs which used in medicine having carbonyl groups which when reduced by reductase enzyme in the body make it have chiral carbon, The compounds which contain benzene ring having stereochemistry due to it have double bond. Mostly drugs having stereochemistry which make them active compounds e.g. phthalazine, quinazoline, quinoxaline and benzimidazole derivatives [19-25].
Literature Review
Quinine
Quinine is a natural alkaloid obtained from cinchona bark used in treatment of malarial disease [26].
Quinine is levo form.
Quinidine
Quinidine also natural alkaloid obtained from cinchona bark from cinchona bark and used in the treatment of arrhythmia [27-29].
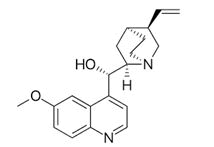
Quinidine is dextro form.
Cyclophosphamide
Cyclophosphamide (cytophosphane) is anticancer agent used I treatment lymphoma, breast cancer, lung cancer…. etc. [30,31].
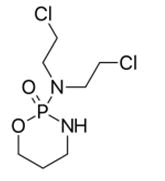
The levo form of cyclophosphamide is more safe than dextro form of which is more toxic [32].
Ketamine
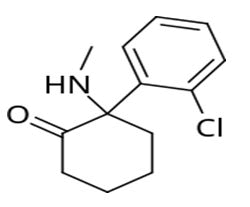
Ketamine is a general anesthetic drug when the drug in dextro form whiles the ketamine in levo form is agitating agent (hallucination) (agitate the patient) [33-38].
Secobarbital
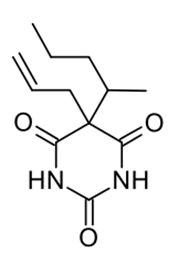
Secobarbital is a member of barbiturates which used as anticonvulsant when it is in dextro for. While the levo form of secobarbital used as general anesthetic agent [39-41].
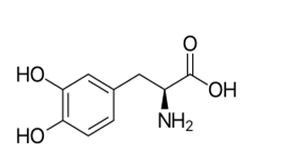
Levodopa
Levodopa (L-Dopa) is the antiparkinsonism when is in levo form which convert into dopamine in the brain.
The dextro form is a granulocytosis and not a cross the blood brain barrier. But the levo form a cross the blood brain barrier because it’s similar to amino acids [42-45].
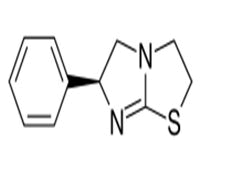
Levamisole
Levamisole is the levo form of tetramisole Which used as anthelmentic agent where the first used was racemic mixture which cause abdominal pain ( mainly due to the dextro form) [46-48].
Thalidomide
Thalidomide theoritically only the inactive S (-) isomer is tratogenic but practically both isomers geno toxic because of its in vivo interconvertion and of its species dependants [49-51].
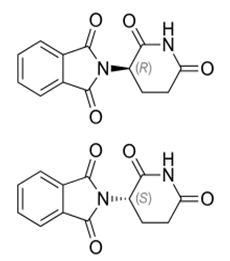
The S isomer in cotrast to the R isomer has been linked to thalidomide tratogenic effects. N.B. the R isomer have not solved the problem of tratogenicity so the tratogenicity due to the numerous chiral and a chiral metabolites of which pharmacologocal and toxocological studies which remain very scarce [52].
Cisplatin
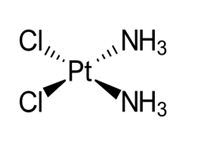 Cisplatin is anticancer agent which more active than transforms [53].
Cisplatin is anticancer agent which more active than transforms [53].
Levoadrenaline
Levoadrenaline synthesised by adrenalmedulla which synthesis it from tyrosine (amino acid) [54].
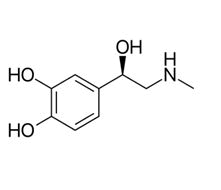
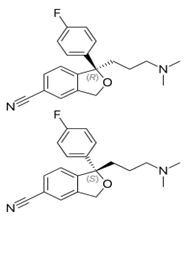
The levo adrenaline is more active than dextro adrenaline.
The (S) (+) form is more than 100 fold potent as selective serotonine reuptake inhebetor than (R.) (-) isomer [55].
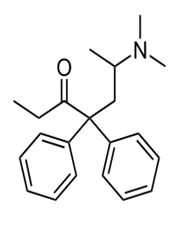
Mithadone
Mithadone is analgesic which acts centrally with high affinity for opioid receptors, has been used to treat dependence resulted from opiate and used to treat the cancer pain. Mithadone is a chiral synthetic compound used in therapy under recemic mixture in humans R (-) mithadone is about (25-50) times more potent as an analgesic than its S (+) antipode [56-59].
Discussion
All drugs which used medicine must be we know if these compounds have chiral carbon or have any form of stereochemistry of them. This knowledge explain and sharing in mechanisms, binding with receptors, pharmacological action. So must be study the stereochemistry in drug design.
Conclusion
The stereochemistry is important for explain the stereo of the compounds which used in medicine for treatment diseases where there is some isomeres (enantumers) may be uneffected, toxic or reveal some undesiraple effects which increase the diseases or suffer from the side effects.
References
- Challener CA (2001) In: Chiral drugs. 1st. Aldershot (England), Overview of chirality, Ashgate Publisher, London: 3–14
- Drayer DE (1993) Drug stereochemistry. Analytical methods and pharmacology. 2nd edition. Wainer IW, editor. Marcel Dekker Publisher, New York: 1–24
- Rentsch KM (2002) The importance of stereoselective determination of drugs in the clinical laboratory. J Biochem Biophys Methods 54:1–9
[Crossref] [Google scholar] [Indexed]
- Walther W, Netscher T (1996) Design and development of chiral reagents for the chromatographic determination of chiral alcohols. Chirality 8:397–401
- Katzung BG (2004) In: Basic and clinical pharmacology. 9th edition. Lange Medical Books/McGraw Hill, New York.
- Borman S (2001) Asymmetric catalysis wins. Chemistry Nobel honors Knowles, Noyori, Sharpless for chiral syntheses. Chem Eng News 79:5-6
- Liu JT, Liu RH (2002) Enantiomeric composition of abused amine drugs: chromatographic methods of analysis and data interpretation. J Biochem Biophys Methods 54:115–146
[Crossref] [Google scholar] [Indexed]
- Wainer IV, Marcotte AA (1993) Sterochemical terms and concepts. An overview. In: Wainer IW, editor. Drug stereochemistry. Analytical methods and pharmacology. (2nd edition), Marcel Dekker Publisher, New York.
- Landoni MF, Soraci A (2001) Pharmacology of chiral compounds: 2-Arylpropionic acid derivatives. Curr Drug Metab 2:37–51
[Crossref] [Google scholar] [Indexed]
- Lee EJ, Williams KM (1990) Chirality Clinical Pharmacokinetic and Pharmacodynamic Considerations. Clin Pharmacokinet 18:339–345
- Cahn RS, Ingold Ck, Prelog V (1956) The specification of asymmetric configuration in organic chemistry. Experienta 12:81–124
[Crossref] [Google scholar] [Indexed]
- Ariens EJ, Soudijn W, Timmermans PBMWM (1983) Stereoselectivity of bioactive agents: general aspects. In: Stereochemistry and Biological activity of Drugs.Oxford: Blackwell Scientific 11–33 [Crossref] [Google scholar] [Indexed]
- Drayer DE (1986) Pharmacodynamic and pharmacokinetic differences between drug enantiomers in human: an overview.Clin Pharmacol Ther 40:125–133
[Crossref] [Google scholar] [Indexed]
- Waldeck B (2003) Three-dimensional pharmacology, a subject ranging from ignorance to overstatements.Pharmacol Toxicol 93:203–210
[Crossref] [Google scholar] [Indexed]
- Davies NM, Teng XV (2003) Importance of chirality in drug therapy and pharmacy practice. Implication for psychiatry.Adv Pharma 1:242–252
- Stoschitzky K, Lindner W, Zernig G (1998) Racemic beta-blockers-fixed combinations of different drugs.J Clin Bas Cardiol 1:15–19
- Barrett A, Cullum C (1968) The biological properties of the optical isomers of propranolol and their own cardiac arrhytmias.Br J Pharmacol 34:43–55
[Crossref] [Google scholar] [Indexed]
- Rahn K, Hawhina A, Kersting F, Planz G (1974) Studies on the antihypertensive action of propranolol in man.Naunyn Schmiedebergs Arch Pharmacol 286:319–323
[Crossref] [Google scholar] [Indexed]
- Stoschitzky K, Lindner W, Egginger G, Brunner F, Obermayer-Pietsch B, et al. (1992) Racemic (R,S)-propranolol versus half-dosed optically pure (S)-propranolol in man at steady state: Hemodynamic effects, plasma concentrations, and influence on thyroid hormone levels.Clin Pharmacol Ther 51:445–453
[Crossref] [Google scholar] [Indexed]
- Wiersinga WM, Touber JL (1997) The influence of ß-adrenoceptor blocking agents on plasma thyroxine and triiodothyronine.J Clin Endocrinol Metab 45:293–298
[Crossref] [Google scholar] [Indexed]
- Harrower ADB, Fyffe JA, Horn DB, Strong JA (1997) Thyroxine and triiodothyronine levels in hyperthyroid patients during treatment with propranolol.Clin Endocrinol 7:41–44
[Crossref] [Google scholar] [Indexed]
- Echizen H, Manz M, Eichelbaum M (1998) Electrophysiologic effects of dextro and levo verapamil on sinus node and AV node function in humans.J Cardiovasc Pharmacol 12:543–546
[Crossref] [Google scholar] [Indexed]
- Satoh K, Yanagisawa T, Taira N (1980) Coronary vasodilatator and cardiac effects of optical isomers of verapamil in the dog.J Cardiovasc Pharmacol 2:309–318
[Crossref] [Google scholar] [Indexed]
- Industry Overview. organised by scientific update. Conferences; An international symposium on chirality; 5-7 September 1999; Cambridge University, Cambridge, UK.
- Patocka J, Dvorak A (2004) Biomedical aspects of chiral molecules.J Appl Biomed 2:95–100
- Jamali F, Mehvar R, Pasutto FM (1989) Enantioselective aspects of drug action and disposition: Therapeutic pitfalls.J Pharm Sci 78:695–715
[Crossref] [Google scholar] [Indexed]
- Sakr HM, Ayyad RR, Mahmoud K, Mansour AM, Ahmed AG (2021) Design Synthesis of Analgesics and Anticancer of Some New Derivatives of-Benzimidazole. J Org Chem 11:144-169
- Mohamed M Khalifa, Helmy M Sakr, Albaraa Ibrahim, Ahmed M Mansour, Rezk R Ayyad (2022) Design and synthesis of new benzylidene-quinazolinone hybrids as potential anti-diabetic agents: In vitro α-glucosidase inhibition, and docking studies. J Mol Struct 1250: 131768
- Abdel-Ghany El-Helby, Helmy Sakr, Rezk Ayyad, Hazem Mahdi, Eissa IH, et al. (2020) Design, synthesis, molecular modeling, in vivo studies and anticancer activity evaluation of new phthalazine derivatives as potential DNA intercalators and topoisomerase II inhibitors.” Bioorganic Chemistry 103:104233
[Crossref] [Google scholar] [Indexed]
- Rezk Ayyad (2012) “Synthesis and biological evaluation of novel iodophthalazinedione derivatives as anticonvulsant agents.” Al-Azhar JPharm Sci 45:1-13
[Crossref] [Google scholar] [Indexed]
- Wagdy M Eldehna, Sahar Abou-Seri, Ahmed ElKerdawy, Rezk R, Hamdy AM, et al. (2016) “Increasing the binding affinity of VEGFR-2 inhibitors by extending their hydrophobic interaction with the active site: Design, synthesis and biological evaluation of 1-substituted-4-(4-methoxybenzyl) phthalazine derivatives.” Eur J Med Chem 113:50-62
[Crossref] [Google scholar] [Indexed]
- El-Helby Abdel Ghany A, Rezk R Ayyad, Helmy M Sakr, Adel S Abdulrahim, El-Adl K, et al. (2017) “Design, synthesis, molecular modeling and biological evaluation of novel dihydrophthalazine-1, 4-dione derivatives as potential anticonvulsant agents.” J Mol Struct 1130:333-351
[Crossref] [Google scholar] [Indexed]
- El-Helby Abdel-Ghany A, Rezk RA Ayyad, Helmy Sakr, Khaled El-Adl, Mamdouh M Ali, et al. (2017) ”Design, Synthesis, Molecular Docking, and Anticancer Activity of Phthalazine Derivatives as VEGFR-2 Inhibitors. Archiv der Pharmazie 350:1700240
[Crossref] [Google scholar] [Indexed]
- El-Helby Abdel-Ghany A, Rezk RA Ayyad, Khaled El-Adl, Hazem Elkady (2019) ”Phthalazine-1, 4-dione derivatives as non-competitive AMPA receptor antagonists: design, synthesis, anticonvulsant evaluation, ADMET profile and molecular docking.” Molecular Diversity 23: 283-298
[Crossref] [Google scholar] [Indexed]
- Eissa Ibrahim H, Ahmed M Metwally, Amany Belal, Ahmed BM Mehany, Ayyad RR, et al. (2019) ”Discovery and antiproliferative evaluation of new quinoxalines as potential DNA intercalators and topoisomerase II inhibitors.” Archiv der Pharmazie 352:e1900123
[Crossref] [Google scholar] [Indexed]
- Ibrahim Mohamed-Kamal, Ashraf A Abd-Elrahman, Rezk RA Ayyad, Khaled El-Adl, Ahmed M, et al. (2013) ”Design and synthesis of some novel 2-(3-methyl-2-oxoquinoxalin-1 (2H)-yl)-N-(4-(substituted) phenyl) acetamide derivatives for biological evaluation as anticonvulsant agents.” Bull Fac Pharm 51:101-111
[Crossref] [Google scholar] [Indexed]
- Elhelby Abdelghany Aly, Rezk Rezk Ayyad, Mohamed Fathallah Zayed (2011) “Synthesis and biological evaluation of some novel quinoxaline derivatives as anticonvulsant agents.” Arzneimittelforschung 61:379-381
[Crossref] [Google scholar] [Indexed]
- El-Helby Abdel-Ghany A, Rezk RA Ayyad, Khaled El-Adl, Alaa Elwan (2017) ”Quinoxalin-2 (1H)-one derived AMPA-receptor antagonists: design, synthesis, molecular docking and anticonvulsant activity.” Med Chem Res 26:2967-2984
- El-Helby Abdel-Ghany A, Rezk RA Ayyad, Mohamed F Zayed, Hamada S Abuelkheer, et al. (2019) “Design, synthesis, in silico ADMET profile and GABA-A docking of novel phthalazines as potent anticonvulsants.” Archiv Der Pharmazie 352:1800387
[Crossref] [Google scholar] [Indexed]
- El-Helby Abdel-Ghany A, Helmy Sakr, Rezk RA Ayyad, Khaled El-Adl, et al. (2018) “Design, Synthesis, In vitro Anti-cancer Activity, ADMET Profile and MolecularDocking of Novel Triazolo [3, 4-a] phthalazine Derivatives Targeting VEGFR-2 Enzyme.” Anticancer Agents Med Chem 18: 1184-1196
[Crossref] [Google scholar] [Indexed]
- Mohd Nassar Ekhlass, Fathy M Abdelrazek, Rezk R Ayyad, Ahmed F El-Farargy (2016) “Synthesis and some reactions of 1-aryl-4-acetyl-5-methyl-1, 2,triazole derivatives with anticonvulsant activity.” Mini-Rev Med Chem 16:926-36
[Crossref] [Google scholar] [Indexed]
- Ayyad Rezk (2014) “Synthesis and anticonvulsant activity of 6-iodo phthalazinedione derivatives.” Al-Azhar J Pharm Sci 50:43-54
[Crossref] [Google scholar] [Indexed]
- El-Helby AA, Ibrahim MK, Abdel-Rahman AA, Ayyad RRA, Menshawy MA, et al. (2009) “Synthesis, molecular modeling and anticonvulsant activity of benzoxazole derivatives.” Al-Azhar J Pharm Sci 40:252-270
- RezkRezk AA, Sakr H, El-Gamal K (2016) Synthesis, modeling and anticonvulsant activity of some phthalazinedione. Am J Org Chem 6:29-38
[Crossref] [Google scholar] [Indexed]
- RezkRezk AA (2008) Synthesis and anticonvulsant activity of tetrabromophthalazinedione. AZ J Pharm Sci 33-45
- El-Helby AA, Ibrahim MK, Amin MA, RezkRezk AA (2022) Synthesis and anticonvulsant activity of phthalazindione derivatives. AZ J Pharm Sci 28:22-35
- Ibrahim MK, AA Abdel-Rahman, RRA Ayyad, K El-Adl, F Elsherbeny, et al. (2010) “Design and synthesis of some novel N-phthalimide derivatives with potential anticonvulsant activity.” Al-Azhar J Pharm Sci 42:305-322
- Abdel-Aziz AA, Abou-Zeid LA, ElTahir KEH, Ayyad RR, El-Sayed MA, et al. (2016) “Synthesis, anti-inflammatory, analgesic, COX-1/2 inhibitory activities and molecular docking studies of substituted 2-mercapto-4 (3H)-quinazolinones.” Eur J Med Chem 121:410-421 Crossref]
- Mohamed MA, Ayyad RR, Shawer TZ, Abdel-Aziz AA, El-Azab AS (2016) “Synthesis and antitumor evaluation of trimethoxyanilides based on 4 (3H)-quinazolinone scaffolds.” Eur J Med Chem 112: 106-113
[Crossref] [Google scholar] [Indexed]
- Hall JH, Hall ES, Wong OT (1992) Cumulated Index Medicus. Anticancer Drug 53:55-62
- Soje-Echaque E, Lim RKS (1962) Inflammation and Anti-inflammatories. Spectrum, New York, USA. J Pharmacol Exp Ther 138:224-228
- Synthesis and anticonvulsant activity of tetrabromophthalazin-1,4-dione derivatives. AZ J Pharm Sci 2008:37
- Ibrahim MK, El-Helby AE, Ghiaty AH, Biomy AH, Abd-El Rahman AA, et al. (2009) Modeling, Synthesis, and antihypeglycemic activity of novel quinazolinones containing sulfonylurea moiety. J Biol Sci 7:40-51
- Modeling, Synthesis, and anticonvulsant activity of some new quinazolinones. AZ J Pharm Sci 2010:41
- Ibrahim MK, Abdel-Rahman AA, Ayyad RR, El-Adl K, Elsherbeny F, et al. (2010) Design and synthesis of some novel N-phthalimide derivatives with potential anticonvulsant activity. Al-Azhar J Pharm Sci 42:305-322
- Rezk Ayyad (2012)Synthesis and Biological Evaluation of novel iodophthalazinedione derivatives as anticonvulsant Agents. AZ j pharm Sci 45:1-13
[Crossref]
- Elhelby AA, Ayyad RR, Zayed MF (2011) Synthesis and Biological Evaluation of some novel quinoxaline derivatives as anticonvulsant Agents. Arzneimittelforschung 61:379-381
- Ibrahim MK, Abd-Elrahman AA, Ayyad RR, El-Adl K, Mansour AM, et al. (2013) Design and synthesis of some novel 2-(3-methyl-2-oxoquinoxalin-1 (2H)-yl)-N-(4-(substituted) phenyl) acetamide derivatives for biological evaluation as anticonvulsant agents. Bulletin of Faculty of Pharmacy, Cairo University 51:101-111
[Crossref] [Google scholar] [Indexed]
- Rezk RA, Ahmed MM (2014) Synthesis and anticonvulsant activit1y of 6-iodophthalazinedione derivatives. Al-Azhar J pharm sci 50:43-54

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences
