ISSN : 0976-8505
Der Chemica Sinica
Spectral, Thermal, X-Ray and Antimicrobial Studies of Newer Tetradentate N2O2 Schiff Base Complexes of First Transition Series
Shyam R Annapure, Achut S Munde and Shantilal D Rathod*
Department of Chemistry, Milind College of Science, Aurangabad, Maharashtra, India
Abstract
Asymmetrical tetradentate complexes of Mn(II), Fe(III), Co(II), Ni(II) and Cu(II) of Schiff bases which are synthesized from o-phenylenediamine, 3-Acetyl-6-methyl-pyran-2, 4-dione and 5-bromo salicylaldehyde have been synthesized. The structures of ligands and complexes are characterized by elemental analysis, magneticsusceptibility, thermal analysis, X-ray diffraction, 1H-NMR, mass, IR, UV-visible spectra, and conductometry. TGA/DSC spectral and kinetic parameter of the complexes was observed keenly. The ligand field parameters have been characterized for Mn(II), Fe(III), Co(II), complexes, which recommend octahedral geometry, and square planar geometry for Ni(II) and Cu(II). The X-ray diffraction data proposes monoclinic crystal system for all five complexes. The ligand and their metal complexes were subjected for antibacterial activity against Escherichia coli and Staphylococcus aureus and fungicidal activity against Trichoderma and Aspergillus Niger.
Keywords
Dehydroacticacid, Tetradentate Schiff base, Thermal analysis, Powder X-ray diffraction, Antimicrobial activity
Introduction
The contribution of Schiff Bases and its complexes with metal in the ocean of coordination chemistry [1], medicinal, analytical, supramolecular chemistry is due to their capability of chelation with variety of metal ions. So researcher and chemist infatuated with the idea of use it in Catalysis [2] or use it as potential antibacterial [3], antifungal [4-6], antitumor [7,8] anti-tuberculosis [9], antioxidant [10], DNA cleavage [11] agents. It is well known fact that asymmetrical Schiff bases with N2O2 donor atoms are Igor toco-ordinate with various metal ions forming stable compound with aliphatic as well as aromatic compound i.e. 1,2-diamines and 3-Acetyl-4-hydroxy-6-methyl-2Hpyran- 2-one. Dehydroacetic acid serves as a precursor for the synthesis of large number of heterocyclic derivatives [12]. In addition the derivatives of DHA are well known to possess potential anti-fungal and anti-microbial properties [13]. After going through literature it was revealed that it is more challenging to prepare asymmetrical Schiff bases of this type [14].
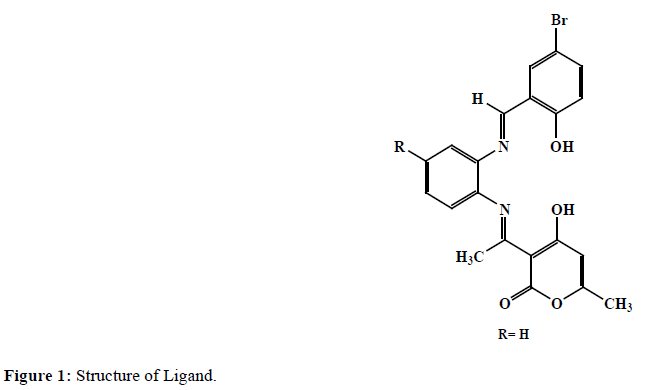
In this paper we are proclaiming our earlier work of tetradentate Schiff bases formed by the reaction of o-phenylenediamine, DHA, and 5-bromo Salicylaldehyde (Figure 1). The complexes of various color of Mn(II), Fe(III), Co(II), Ni(II) and Cu(II) with this tetradentate ligands were synthesized and characterized.
Materials and Methods
Merck was the supplier for all reagents and solvents. DHA, o-phenylenediamine, and 5-bromoSalicylaldehyde of AR grade were used for synthesis of ligand. AR grade metal chlorides were also used for the formation of the complexes.
Synthesis of ligand
There are several methods to prepare Schiff bases as, beginning from Hugo Schiff. L was prepared by using reported methods with some alteration [15]. It’s a two-step synthesis; in the first step mono-Schiff base compound was prepared by refluxing 50 ml solution of (10 mmol) of DHA and (10 mmol) o-phenylenediamine in absolute ethanol for about 3 hours. The progress of reaction was monitored via thin layer chromatography. The resulting mono Schiff base thus formed was then refluxed with 10 mmol of 5-bromo Salicylaldehyde to synthesis final product. Product was then cooled at room temperature and collected by filtration, followed by recrystallization in super dry ethanol. (Yield: 75%).
Synthesis of metal complexes
Metal complexes were prepared by mixing a stoichiometric ratio (1:1) by dissolving in methanol. The ligand (0.01 mol) and metal chloride (0.01 mol) are mixed in hot condition with continuous stirring [16]. The mixture was heated at reflux for about 3 to 4 hours. On cooling, the volume of reaction mixture is reduced to half, then colored solid metal complex is appeared. Thus obtained solid metal complex was purified by petroleum ether and dried over vacuum desiccator (yield: 75%).
Physical measurement
The CHN analysis was carried out on Thermo Scientific (FLASH 2000) CHN elemental analyser. 1H-NMR spectra of ligand were recorded on FT NMR spectrometer (400 MHZ) model Advance-II (Bruuker) in CDCl3 as a solvent using tetramethylsilane as internal standard. IR study has been carried out on Perkin Elmer-Spectrum RX-I FTIR spectrometer using KBr pellets. These three facilities are availed by SAIF, Punjab University Chandigarh. The TGA/DSC and XRD were recorded on TA Inc. SDT-2790 and Pan analytical X’Pert Pro respectively. All electronic absorption spectra of the complexes and ligand were chronicled on Shimadzu 1800 spectrometer. Molar conductance of complexes was probed on Elico CM 180 conductivity meter using 10-3 M solution in DMF. Magnetic susceptibility of the metal complexes is investigated on a Guoybalance at room temperature using Hg[Co(SCN)4] as a calibrator.
Results and Discussion
Table 1 indicates physical characteristics, as CHN, mp, color and molar conductance data of ligand and metal complexes. The data shows equimolar stoichiometry (metal:ligand) and satisfying general formula [ML(H2O)2], where M=Mn(II), Fe(III), Co(II) and [ML], where M=Ni(II ) and Cu(II). The study of magnetic properties revel those two types of geometry for the complexes. One octahedral for Mn(II), Fe(III) and Co(II) at room temperature with two water molecules coordinated to metal ion. Existence of two coordinated water molecules was further confirmed by weight loss before 270˚C in TGA-DSC analysis. Second square-planar geometry for Ni(II), Cu(II) complexes at room temperature.
| Compound Molecular formula | Mol.Wt. | M.P /Decomp Temp. 0C |
Color | Molar conduc. Mho cm2 mol-1 |
Found (calculated) | |||
|---|---|---|---|---|---|---|---|---|
| C | H | N | M | |||||
| (H2L) C21H17N2O4Br |
442.10 | 189 | Dark Yellow |
------- | 56.71 (57.16) |
3.69 (3.88) |
6.87 (6.35) |
----- |
| [LMn(H2O)2] | 496.21 | 234 | Brown | 19.20 | 50.22 (50.83) |
2.65 (2.45) |
5.85 (5.65) |
11.55 (11.07) |
| [LFe(H2O)2] | 497.12 | 260 | Reddish Brown |
42.21 | 50.23 (50.74) |
2.63 (2.55) |
5.98 (5.64) |
11.86 (11.23) |
| [LCO(H2O)2] | 500.21 | 223 | Faint Red |
12.40 | 50.75 (50.42) |
2.95 (2.55) |
5.85 (5.60) |
12.11 (11.78) |
| [LNi] | 499.97 | 220 | Faint Red |
21.20 | 50.89 (50.45) |
3.12 (2.60) |
5.88 (5.60) |
12.10 (11.74) |
| [LCu] | 504.82 | 237 | Green Brown |
18.10 | 50.22 (49.96) |
2.85 (2.55) |
5.43 (5.55) |
12.45 (12.59) |
Table 1: Physical characterization, analytical and molar conductance data of compounds.
1H-NMR spectra of ligand
The 1H NMR spectra of free ligand in CDCl3 at room temperature shows the following signals. 2.07 δ (s, 3H, C6- CH3), 2.13 δ (s, 3H, N=C-CH3), 5.83 δ (s, 1H, C5-H), 6.73-7.04 δ (m, aromatic protons), 8.96 δ (s, 1H, N=C-H), 9.98 δ (phenolic (-OH) hydrogen of phenyl ring) and 15.89 δ (s, 1H, enolic OH of DHA moiety) [12,17,18].
FTIR spectra
The IR data of ligand (H2L) and its metal complexes are listed in Table 2. It depict prominent bands at 3296, 1689, 1656, 1360 and 1212 cm-1 assignable to υ OH, υ C=O (lactone carbonyl), υ C=N (azomethine), υ C-N (aryl azomethine) and υ C-O (phenolic) stretching modes respectively [19,20]. The presence of a strong broad band in the 3296 cm-1 region, in the spectra of the ligand, which is not observed in complexes elucidates coordination of phenolic oxygen to the metal ion by deprotonation [21]. Resulting up swing to an extent of 40 cm-1 to 60 cm-1 in the υ C-O (phenolic) band [22]. This shift further confirms the involvement of the enolic oxygen in C-O-M bond [23]. Chelation by nitrogen of azomethine (C=N) is confirmed by observing band at 1656 cm-1 in the spectra of ligand, which find at lower frequency 1603 cm-1 to 1632 cm-1 when complex formed [24]. This change can be supported by transfer of electrons from nitrogen to the vacant d-orbitals of the metal [25]. Finding new bands in the 447-565 and 410-480 cm-1 regions confirms the M-O and M-N bonding respectively [26]. No any change in skeletal vibrations (C=C) upon complexation. The presence of coordinated water is confirmed by the appearance of strong band in the 3027 cm-1 to 3096 cm-1 region in case of Mn(II), Fe(III) and Co(II),which is also supported by appearance of non-ligand band in 825 cm-1 to 846 cm-1 region, quoted for rocking mode of water [21,27].
| Compound | IR band frequency (cm-1) | |||||||
|---|---|---|---|---|---|---|---|---|
| ν (OH) | ν (C=O) | ν (C=N) | C=C | C-N | C-O | M-O | M-N | |
| L | 3296 | 1689.3 | 1656.7 | 1566.8 | 1360 | 1212 | - | - |
| Mn-L | 3027 | 1687.2 | 1626 | 1517.7 | 1330 | 1256 | 526 | 480 |
| Fe-L | 3096 | 1682.1 | 1632 | 1567.1 | 1325 | 1257 | 565.2 | 478.2 |
| CO-L | 3088 | 1679.0 | 1624 | 1566.0 | 1321 | 1285 | 550.8 | 447.1 |
| Ni-L | 3025 | 1684.0 | 1620 | 1566.0 | 1327 | 1260 | 447.3 | 410 |
| Cu-L | 3284 | 1681.2 | 1603 | 1566.0 | 1321 | 1267 | 525.1 | 477.1 |
Table 2: IR data of ligand and metal complexes.
Magnetic susceptibility and electronic absorption spectra
The electronic absorption spectrum of Mn(II) complex contains three bands at 14727,24691 and 26455 cm-1 assignable to the transitions 6A1g → 4T1g, 6A1g → 4T2g and charge transfer respectively. Magnetic moment value 5.84 BM matches with standard value (5.92 BM) corresponds octahedral geometry for Mn(II) complex [28,29]. The electronic absorption spectra of Fe(III) complex show three strong bands at 12787, 18621 and 34364 cm-1 which may be assigned to the transitions 6A1g → 4T1g(4D), 6A1g → 4T1g and charge transfer respectively. Electronic transitions together with magnetic moment value 5.83 BM indicates high spin octahedral geometry for Fe(III) complex [30]. Co(II) complex show three bands at 10660, 18796 and 26881 cm-1 which may be attributed to the transitions 4T1g → T2g(F), 4T1g → 4A2g(F) and charge transfer respectively. Electronic transitions along with magnetic moment value 4.55 B.M. suggest high spin octahedral geometry for Co(II) complex [30,31]. The octahedral geometry is further supported by ratio υ2/ υ1=1.750 which is close to the value expected for octahedral geometry. The electronic absorption spectra of Ni(II) complex consists of two bands at about 17361 cm-1 and 26385 cm-1 assignable to 1A1g → 1T2g and charge transfer transitions respectively. Observed electronic transitions, the diamagnetic nature and red color of the complex suggests square planar geometry for Ni(II) complex [31,32] Cu(II) show three bands at 16366, 263852 and 37313 cm-1 assignable to the transitions 2B1g → 2A1g and two intra ligand charge transfer bands. These electronic transitions and observed 1.80 BM magnetic moment value suggests square-planar geometry around Cu(II) [13,33]
Thermal analysis
The TG/DSC analysis of all Mn(II), Fe (III), Co(II), Ni(II) and Cu(II) complexes was done from ambient temperature to 1000°C in nitrogen atmosphere using α-Al2O3 as reference. The TG curve of Mn(II) complex show first mass loss 6.39% (calcd.6.40%) in the range 160-230°C and an endothermic peak in this region ΔT min=200°C, indicate removal of two coordinated water molecules. The second step slow decomposition from 250°C to 1000°C with 14% mass loss. This can be further confirmed by observing broad exotherm in DSC with ΔT max=378°C indicates that the complex is thermally quite stable. The thermal profile of Fe(III) complex shows mass loss 4.7% (calcd.4.64%) in the range 160°C-230°C and an endothermic peak in this region ΔT min=217°C indicates loss of two coordinated water molecules [34]. The anhydrous complex first show slow decomposition from 230°C to 645°C with mass 32.66% (calcd.32.44%) loss and a broad exotherm ΔT max=360°C in DSC may be attributed to removal of non-coordinated part of ligand. The second step decomposition is sharp from 645°C to 675°C with mass loss of 14.50% (calcd. 14.85%) a sharp endotherm in DTA at 650°C is observed for this step. The third step decomposition is from 675°C to 820°C with 19% mass loss. The mass of the final residue 8.2% does not corresponds to any stoichiometry of end product. The thermogram of Co(II) complex show mass loss 6.6% (calcd.6.8%) in the range 160°C to 240°C and an endothermic peak in this region ΔT min=230°C, indicates loss of two coordinated water molecules [13,30]. The anhydrous complex first show slow decomposition in 240°C to 500°C range with 27% mass loss (calcd.27.01%) and a broad exotherm ΔTmax=316°C in DSC may be attributed to removal of non-coordinated part of ligand. The second step decomposition at 500°C to 850°C, with mass loss of 53.30% (calcd.53.08%) corresponds to decomposition of coordinated part of ligand. A broad endotherm in DSC is observed for this step. The mass of the final residue corresponds to stable CoO with mass 13.36% (calcd.13.85%).
The TG curve of Ni(II) complex, show three step decomposition. No mass loss up to 230°C indicates absence of coordinated water in these complexes. The first step from 230°C to 350°C, with a mass loss 11.57% (calcd. 11.60%), an endothermic peak ΔT min=244.62°C in DSC may be attributed to the decomposition of non-coordinated part of ligand. The sudden decomposition in second step from 350°C to 400°C with mass loss 10.51% (calcd.10.65%), an endothermic peak ΔT min=359.29°C in DSC. Third decomposition slow and starts from 400°C to 800°C with mass loss 52.99%, an exothermic peak in DSC at ΔT max=423.73°C may corresponds to removal of coordinated part of ligand. The mass of the final residue 11% does not corresponds to any stoichiometry of end product.
In the TG curve of Cu(II) complexes no mass loss up to 250°C indicates absence of coordinated water in these complexes [35]. In the TG curve of Cu(II) complex, the first step of decomposition from 250°C to 350°C, with a mass loss 26.70% (calcd. 25.25%), an exothermic peak ΔT max=265.47°C in DSC may be attributed to the removal of non-coordinated part of ligand. The second slow step from 350°C to 650°C with mass loss 21.50% (calcd.21.46%),an exothermic peak ΔT max=438.90°C. The third step starts from 650°C to 850°C with mass loss 39.43% (calcd.40.46%) a broad endotherm is observed in same region in DSC corresponds to decomposition of coordinated part of ligand. The mass of the final residue corresponds to stable CuO, 10.5% (calcd.10.82%).
Kinetic calculations
The kinetic and thermodynamic parameters viz ΔG ( free energy change), ΔS, z ( pre-exponential factor), Ea and n (order of reaction), together with correlation coefficient (r) for non-isothermal decomposition of metal complexes have been determined by Horowitz-Metzer (HM) approximation method [36] and Coats-Redfern integral method [37] The data is arranged in Table 3. The results show that the values obtained by two methods are analogous. Low values of Eaof the complexes are indicating the autocatalytic effect of metal ion after thermal decomposition.
| Complex | Step | n | Method | Ea (kJ)mol-1 |
Z S-1 |
∆S JK-1mol-1 |
∆G kJ mol-1 |
Correlation coefficient (r) |
|---|---|---|---|---|---|---|---|---|
| Mn (II) | I | 1.35 | HM CR |
35.14 34.08 |
58320 172113 |
-159.28 -111.99 |
41.63 41.93 |
0.9997 0.9986 |
| II | 1.35 | HM CR |
24.08 23.99 |
61868 362280 |
-132.69 -89.69 |
36.73 32.50 |
0.9989 0.9980 |
|
| Fe (III) | I | 0.55 | HM CR |
33.99 31.54 |
44568 47183215 |
-103.76 -104.61 |
42.20 39.81 |
0.9999 0.9987 |
| II | 0.55 | HM CR |
40.47 38.83 |
73267 35088264 |
-120.17 -91.63 |
55.32 50.16 |
0.9999 0.9999 |
|
| CO (II) | I | 0.51 | HM CR |
32.42 31.88 |
53248 75580 |
-129.69 -85.00 |
47.98 42.08 |
0.9980 0.9989 |
| Ni (II) | I | 0.51 | HM CR |
53.89 51.72 |
66843 2044177 |
-159.16 -130.71 |
66.48 62.06 |
0.9990 0.9979 |
| II | 0.39 | HM CR |
28.03 27.65 |
42848 625548707 |
-132.92 -85.97 |
42.86 37.24 |
0.9999 0.9999 |
|
| Cu (II) | I | 1.13 | HM CR |
62.07 59.74 |
124015 112875 |
-152.34 -153.12 |
70.37 69.73 |
0.9981 0.9952 |
| II | 0.35 | HM CR |
17.25 17.56 |
29812 425170645 |
-142.44 -87.17 |
29.72 25.19 |
0.9992 0.9995 |
Table 3: The kinetic parameter of metal complexes calculated by the methods Horowitz-Metzger (HM) and Coats-Redfern (CR).
Powder X-ray diffraction
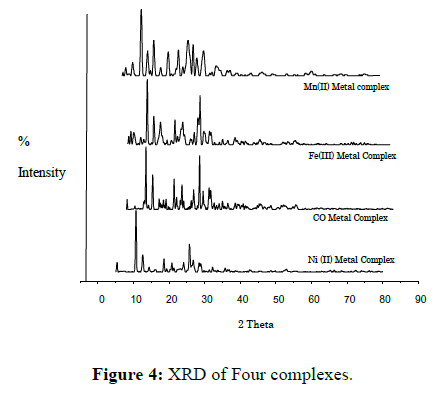
Scanning of X-ray diffractogram of Mn(II), Fe(III), Co(II), Ni(II) and Cu(II)metal complexes of L is done at wavelength 1.543 Å in the range 5° to 100°. The X-ray diffraction pattern of these complexes compared with major peaks of relative intensity greater than 10% has been indexed to their hkl value by using computer program [38]. The diffractogram of Mn(II) complex of L had nine reflections with maxima at 2θ=8.123° corresponding to d value 10.87616Å. The unit cell of Mn(II) complex of L yielded values of lattice constants, a=14.69362 Å, b=5.101341 Å, c=12.86531 Å and unit cell volume V=929.98359 Å3. The diffractogram of Fe(III) complex of L shows eight reflections with maxima at 2θ=10.673° corresponding to d value 8.28255 Å. The unit cell of Fe(II) complex of L yielded values of lattice constants, a=7.874721 Å, b=16.62674 Å, c=4.850123 Å and unit cell volume V=625.59328 Å3. The diffractogram of CO(II) complex of L had eight reflections with maxima at 2θ=5.924° corresponding to d value 14.90744 Å. The unit cell parameters of Co(II) complex of L yielded values of lattice constants, a=15.90439Å, b=7.102246 Å, c=8.499650 Å and unit cell volume V=897.70014 Å3. The diffractogram of Ni(II) complex of L had ten reflections with maxima at 2θ=10.533° corresponding to d value 8.39194 Å. The unit cell of Ni(II) complex of L yielded values of lattice constants, a=14.44839 Å, b=8.387201 Å, c=7.482108 Å and unit cell volume V=897.24919 Å3. The diffractogram of Cu(II) complex of L had Fourteen reflections with maxima at 2θ=10.598° corresponding to d value 8.35818 Å. The unit cell of Cu(II) complex of L yielded values of lattice constants, a=11.67385 Å, b=9.076698 Å, c=8.643076 Å and unit cell volume V=885.63313 Å3. In respect of these cell parameters, the condition such as a ≠ b ≠ c and α= γ= 90° ≠ β required for sample to be monoclinic were tested and found to be satisfactory. Hence it can be concluded that Mn(II), Fe (III), Co(II), Ni(II) and Cu(II) complex of L has monoclinic crystal system. Density values of the complexes were determined practically by using specific gravity method [39] and found to be 2.5002, 2.3560, 2.8890, 2.9568 and 3.2345 gcm-3 for Mn(II), Fe(III) ,Co(II), Ni(II) and Cu(II) complexes respectively. Where theoretical density found to be 2.5174, 2.3346, 2.8965, 2.9789 and 3.2378 gcm-3 for respective complexes, and find near to experimental value. By using experimental density values, molecular weight of complexes, Avogadro’s number and volume of the unit cell were computed.
Antimicrobial activity
Ligand and metal complexes are subjected for antimicrobial activity against bacteria such as Escherichia coli and Staphylococcus aureus by paper disc plate method [36]. The compounds were tested at the concentration 500 ppm and 1000 ppm in DMF, considering Ciproflaxin as standard (Table 4). Mycelia dry weight method is used for fungicidal activity, compounds were tested against Trichoderma and Aspergillus Niger [40]. The activity is tested at 250 and 500 ppm in DMF and depicted in (Table 5) with comparison with standard.
| Test Compound | Inhibition Zone (mm) | |||
|---|---|---|---|---|
| E.Coli | Staphylococcus | |||
| 500 ppm | 1000 ppm | 500 ppm | 1000 ppm | |
| Ciproflaxin | 29 | 32 | 31 | 35 |
| (H2L) | 11 | 14 | 11 | 14 |
| [LMn(H2O)2] | 11 | 15 | 11 | 16 |
| [LFe(H2O)2] | 11 | 15 | 11 | 16 |
| [LCO(H2O)2] | 13 | 17 | 11 | 16 |
| [LNi] | 15 | 18 | 12 | 16 |
| [LCu] | 19 | 21 | 16 | 19 |
Table 4: Antibacterial activity of compounds.
| Test Compound | Aspergillus Niger | Trichoderma | ||
|---|---|---|---|---|
| 250 ppm | 500 ppm | 250 ppm | 500 ppm | |
| Control | 79 | 79 | 70 | 70 |
| (H2L) | 47(41) | 19(76) | 29(59) | 17(76) |
| [LMn(H2O)2] | 36(54) | 14(82) | 26(63) | 05(93) |
| [LFe(H2O)2] | 37(53) | 17(78) | 27(61) | 08(89) |
| [LCO(H2O)2] | 36(54) | 13(84) | 21(70) | 02(97) |
| [LNi] | 31(61) | 10(87) | 12(83) | 02(97) |
| [LCu] | 34(57) | 08(90) | 10(86) | 01(99) |
Table 5: Yield of Mycelial dry weight in mg (% inhibition).
Observing Tables 4 and 5, conclusion made that the inhibition by metal complexes is more than a ligand [33,36,40]. Solubility of metal complexes in organic solvents increases its activity [36]. Hydrogen bonding with active center of cell may responsible for enhanced activity [41,42].
Conclusion
In present investigation we report synthesis of ligand and its transition metal complexes. Spectral study suggests that azomethine nitrogen and phenolic oxygen are involved in the coordination with metal ions (Figure 2). Proposing octahedral geometry for Mn(II), Fe(III) and Co(II), complexes, and square- planar geometry for Ni(II) and Cu(II) complexes. It is concluded that the ligand is dibasic in nature and ONNO tetradentate metal complexes are biologically active and show enhanced antimicrobial activities compared to its free ligand (Figure 3). The XRD reveals monoclinic crystal system for all Mn(II), Fe (III), Co(II), Ni(II) and Cu(II) complexes. Thermal study predicts thermal behaviour of complexes (Figure 4).
Acknowledgement
The Authors are thankful to Principal Milind College of Science, for providing laboratory facility, and SAIF Punjab University, Chandigarh for providing CHN, IR, 1HNMR, Mass and XRD facility. Also grateful to USIC, Shivaji University, Kolhapur for providing TGA-DSC facility, and Head Dept. of Biotechnology Milind college of science, Aurangabad, for biological activity.
References
- AL Alousi A SH, Shehata MR, Shoukry MM (2008) Coordination properties of dehydroacetic acid - binary and ternary complexes. J Coord Chem 61: 1906-1916.
- Gupta KC, Sutar AK (2008) Catalytic activities of schiff base transition metal complexes. Coord Chem Rev 252: 1420-1450.
- Hasi QM, Fan Y, Yao XQ (2016) Synthesis, characterization, antioxidant and antimicrobial activities of a bidentate Schiff base ligand and its metal complexes. Polyhedron 109: 75-80.
- Debnath S, Mallareddy V, Manjunath SY, Saleshier MF (2010) Conventional and Microwave assisted synthesis of New Pyran, Cyanopyran Schiffs bases and their antimicrobial activities. Int J Pharm Sci Nanotech 3: 1153-1157.
- Venkatesh P (2011) Synthesis, characterization and antimicrobial activity of various schiff bases complexes of Zn(II) and Cu(II) ions. AJPHS 1: 8-11.
- Prasanna MK, Pradeep KK (2013) Synthesis, characterisation and antimicrobial studies of transition metal complexes of 2- hydroxy-5-methoxy benzldehydeisonicot- inoylhydrazone. Res J Chem Environ 17: 61-67.
- Mohsen MK, Ali HI, Manal MA, Mohamed NA, SolimanAMM (2010) Synthesis, antitumor activity and molecular docking study of novel Sulfonamide-Schiff's bases, thiazolidinones, benzothiazinones and their C-nucleoside derivatives. Eur J Med Chem 45: 572-580.
- Shaker N, Fatma H, El-Salam A, El-Sadek BM, Kandeel EM, et al. (2011) Anionic Schiff base amphiphiles: Synthesis, surface, biocidal and antitumor activities. J Am Sci 7: 427-236.
- Patila SA, Manjunatha M, Kamblea UV, Badami PS (2011) Synthesis, spectral characterization and biological evaluation of Co(II), Ni(II), Cu(II) and Mn(II) metal complexes of novel Isatin schiff base ligand. Der Phar Chem 3: 97-108.
- Sashidhara KV, Rosaiah JN, Bhatia G, Saxena JK (2008) Novel keto-enamine Schiffs bases from 7-hydroxy-4-methyl-2-oxo-2H-benzo [h] chromene-8, 10-dicarbaldehyde as potential antidyslipidemic and antioxidant agents. Eur J Med Chem 43: 2592-2596.
- Patel MN, Patel SH, Pansuriya PB (2011) DNA binding and cleavage by dinuclear nickel(II) complexes with neutral bidentate ligands and ciprofloxacin. Med Chem Res 20: 1371-1384.
- Gupta AK, Pal R (2015) Dehydroacetic acid based schiff’s bases and their metal complexes: a review. World J Pharm Pharmaceut Sci 4: 386-425.
- Abdallah SM, Zayed MA, Gehad G (2010) Arabian J Chem 3: 103-113.
- HafeezUllah, Wattoo FH, SarwarWattoo MH (2012) Synthesis, spectroscopic characterization and antibacterial activities of three Schiff bases derived from dehydroacetic acid with various substituted anilines. Turk J Biochem 37: 386-391.
- Wenling Q, Long S, Panunzio M, Biondi S (2013) Schiff bases: a short survey on an evergreen chemistry tool. Molecules 18: 12264-12289.
- Mane PS, Shirodkar SG, Arbad BR and Chondekar TK (2001) Synthesis and characterization of manganese(II), cobalt(II), nickei(Il), and copper(II) complexes of Schiff base derivatives of dehydroacetic acid. Indian J Chem 40A: 648-651.
- Gupta AK, Pal R, Beniwal V (2015) Novel dehydroacetic acid based hydrazone schiff's base metal complexes of first transition series: Synthesis and biological evaluation study. World J Pharm Pharmaceut Sci 4: 990-1008.
- Mangamamba T, Ganorkar MC and Swarnabala G (2014) Characterization of complexes synthesized using schiff base ligands and their screening for toxicity two fungal and one bacterial species on rice pathogens. Int J Inorg Chem 2014: 1-22.
- Pal R, Kumar V, Gupta AK, Beniwal V, Gupta GK (2014) Synthesis, characterization, and DNA cleavage study of dehydroacetic acid based tridentate Schiff's base and its metal complexes of first transition series. Med Chem Res 23: 4060-4069.
- Shelke VA, Jadhav SM, Shankar SG, Munde SC, Chondekar TK (2011) Synthesis and Characterization of Tetradentate N2O2 Schiff Base Ligand and its Rare Earth Metal Complexes. J Korean Chem Soc 55: 436-443.
- Ejidike IP, Ajibade PA (2015) Synthesis, Characterization and Biological Studies of Metal(II) Complexes of (3E)-3-[(2-{(E)-[1-(2,4-Dihydroxyphenyl) ethylidene]amino}ethyl)imino]-1-phenylbutan-1-one Schiff Base. Molecules 20: 9788-9802.
- Jadhav SM, Shelke VA, Munde AS, Shankarwar SG, Patharkar VR, et al. (2010) Synthesis, characterization, potentiometry, and antimicrobial studies of transition metal complexes of a tridentate ligand. J Coord Chem 63: 4153-4164.
- Chitrapriya N, Kamatchi TS, Zeller M, Leea H, Natarajanb K (2011) Synthesis, spectroscopic, crystal structure and DNA binding of Ru(II) complexes with 2-hydroxybenzoic acid [1-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-ethylidene]-hydrazide. Spectrochim Acta 81: 128-134.
- Saini RP, Kumar V, Gupta AK, Gupta GK (2014) Synthesis, characterization and antibacterial activity of a novel heterocyclic Schiff’s base and its metal complexes of first transition series. Med Chem Res 23: 690-698.
- Rao PV, Ashwini K, Ammani S (2007) Synthesis and characterization of transition metal complexes derived from some biologically active furoic acid hydrazones. Bull Chem Soc Ethiop 21: 63-73.
- Miyasaka H, Saitoh A, Abe S (2007) Magnetic assemblies based on Mn(III) salen analogues. Coord Chem Rev 251: 2622-2664.
- Osowole AO, Kolawole GA, Obasola E, Fagade OE (2005) Synthesis, physicochemical and biological properties of nickel(II), copper(II) and zinc(II) complexes of an unsymmetrical tetradentate Schiff-base and their adducts. Synth React Inorg M 35: 829-836.
- Jadhav SM, Shelke VA, Shankarwar SG, Munde AS, Chondhekar TK (2014) Synthesis, spectral, thermal, potentiometric and antimicrobial studies of transition metal complexes of tridentate ligand. J Saudi Chem Soc 18: 27-34.
- Patange VN, Arbad BR (2011) Synthesis, spectral, thermal and biological studies of transition metal complexes of 4-hydroxy-3-[3-(4-hydroxyphenyl)-acryloyl]-6-methyl-2H-pyran-2-one. J Serb Chem Soc 76: 1237-1246.
- Munde AS, Jagdale AN, Jadhav SM, Chondhekar TK (2009) Synthesis and characterization of some transition metal complexes of unsymmetrical tetradentate Schiff base ligand. J Korean Chem Soc 53: 407-414.
- Batra N, Devi J (2015) Synthesis of metal based chemotherapeutic agents derived from chloro-acetic acid[1-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-ethylidene]-hydrazide. J Chem Pharm Res 7: 183-189.
- Borde VL, Nagolkar BB, Shankarwar SG, Shankarwar AG (2015) Synthesis, spectral, thermal, potentiometric and antimicrobial studies of transition metal complexes of tridentate ligand. Res J chem sci 5: 19-23.
- Munde AS, Shelke VA, Jadhav SM, Kirdant AS, Chondhekar TK (2012) Synthesis, characterization and antimicrobial activities of some transition metal complexes of biologically active asymmetrical tetradentate ligands. Adv Appl Sci Res 3: 175-182.
- Maurya RC, Malik BA, Mir JM, Vishwakarma PK, Rajak DK, et al. (2015) Nickel (II) complexes of ONS donor Schiff base ligands: synthesis, combined DFT-experimental characterization, redox, thermal, and in vitro biological investigation. J Coord Chem 68: 2902–2922.
- Dash DC, Panda AK, Jena P, Patjoshi SB, Mahapatra A (2002) Synthesis of some transition metal complexes with 4-(phenyl/p-bromophenyl)thiazolylhydrazone of o-anisaldehyde. J Indian Chem Soc 79: 48-50.
- Cruickshank R, Duguid JP, Marion BP, Swain RHA (1975) Medicinal Microbiology, (Twelfth ed) Churchill Livingstone, London pp.196-202.
- Shelke VA, Jadhav SM, Patharkar VR, Shankarwar SG, Munde AS, et al. (2012) Synthesis, spectroscopic characterization and thermal studies of some rare earth metal complexes of unsymmetrical tetradentate Schiff base ligand. Arab J Chem 5: 501-507.
- Carvajal JR, Roisnel T (2004) WinPLOTR, A graphic tool for powder diffraction, Laborataireleonbrillouin (ceal/enrs) 91191 gif suryvettecedex, France.
- Shoemaker DP, Garland CW (1989) Experiments in physical chemistry. (5th edn) McGraw-Hill International Edition, New York, pp. 17-27.
- Shelke VA, Jadhav SM, Shankarwar SG, Chondekar TK (2013) Synthesis, spectroscopic characterization and antimicrobial activities of some rare earth metal complexes of biologically active asymmetrical tetradentate ligand. J Chem Sci and Tech 2: 61-69.
- CleitonM.da Silva, DanielL.da Silva, LuziaV. Modolo (2011) Schiff bases: A short review of their antimicrobialactivities. J Adv Res 2: 1-8.
- Munde AS, Jagdale AN, Jadhav SM, Chondhekar TK (2010) Synthesis, characterization and thermal study of some transition metal complexes of an asymmetrical tetradentate Schiff base ligand. J Serb Chem Soc 75: 349-359.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences