Some Morphological and Biochemical Changes in the Liver of Adult Wistar Rats Following Lead Acetate Exposure
Ajibade AJ1*, Akinola OT1 and Kehinde BD2
1Department of Anatomy, Lautech Ogbomoso University, Nigeria
2Department of Biochemistry, Lautech Ogbomoso University, Nigeria
- *Corresponding Author:
- Ajibade AJ
Department of Anatomy, Lautech Ogbomoso University
Nigeria
Tel: 23408067881598
E-mail: adeshinaajibade@yahoo.co.uk
Received Date: May 14, 2021; Accepted Date: May 28, 2021; Published Date: June 04, 2021
Citation: Ajibade AJ, Akinola OT, Kehinde BD (2021) Some Morphological And Biochemical Changes In The Liver Of Adult Wistar Rats Following Lead Acetate Exposure. J Anat Sci Res Vol.4 No.4:1.
Abstract
Lead is one of the toxic metals that have been known to be a major environmental pollutant across the world which has been implicated in the etiology of diverse diseases associated with metallic intoxication. Lead is toxic to virtually all organs of the body and has been shown to have significant debilitating effects on the nervous, renal, hepatic and hematopoietic systems. The liver is considered as one of the target organs affected by lead toxicity owing to its site of storage after exposure. Lead could be found in food, drinking water and commercial products. This study investigated the histomorphological effect of lead on the liver of adult wistar rats.
Thirty-six adult wistar rats of both sexes weighing 120 g-250 g were randomly separated into four groups; group A, B, C and D with each group containing 9 rats. Group A rats which served as the control, was maintained on distilled water and standard feed for 28 days, while group B,C and D (lead-treated groups) were treated with 0.09 mg/kg, 0.18 mg/kg and 0.2 mg/kg of lead acetate respectively for 28 days. The lead acetate solution was administered orally on daily basis. The weights of the wistar rats were recorded on weekly basis (before and during the weeks of administration).On the 29th day, the wistar rats in group A,B,C and D were sacrificed by cervical dislocation ,blood was collected through cardiac puncture and used for biochemical analysis (ALP,AST and ALT ), then the liver of each rat was removed and weighed immediately using a sensitive balance and fixed in 10% formol saline, the tissue was processed and sectioned at 5 um and stained with Hematoxylin and Eosin for histological study.
Results showed that the mean body weights of the wistar rats decreased insignificantly (p>0.05) in group B and C when compared to control group A. The body weights of rats in group D decreased significantly (p<0.05) when compared with the control group. The mean liver weights of rats in lead-treated group B showed an insignificant decrease (p>0.05) when compared to control group. Liver weights of rats in Group C showed a significant increase (p<0.05) when compared to the control group. A significant decrease (p<0.05) in the liver weights of rats in group D was observed when compared to the control group. In biochemical analysis, there was statistically significant increase (p<0.05) in the level of ALP, AST and ALT in all the lead-treated groups (B, C and D). Histological study of the liver revealed that the liver parenchyma appeared distorted and degenerated in a dose dependent manner in the lead-treated groups compared with the control which showed normal hepatic histo-architecture.
Keywords
Lead acetate; Liver; ALT; Distortion; ALP; Histomorphology; AST
Introduction
Lead is a common pollutant encountered in every part of the eco-system. It is naturally found in the earth crust as inorganic or organic compound [1]. It is a divalent metal which is grey in colour, soft and malleable. Lead can be used for a variety of things because of its peculiar chemical properties and its poor ability to conduct electricity [2]. It is used in water pipes, as antiknock in petroleum in cosmetic industries etc. It gains access into the environment through the soil, water, air and food [3,4]. Lead poisoning is a global health problem but it is not very much recognized in some African countries. Its exposure is mainly through the gastrointestinal and respiratory systems. The lead that has been taken in, is conjugated on the liver and passed to the kidney, where a small quantity is excreted in urine and the rest accumulates in various body organs. This affects many biological activities at the molecular, cellular and intercellular levels and can result in morphological alterations that could remain even after the lead levels have fallen [5]. It is passed unto animals and man through the food chain. It could be toxic to the animal or man based on its chemical form administered, how often and how long the administration was and the route of administration. Most of the human body is affected by lead if exposure to the metal exceeds the tolerable or bearable level. All age groups are susceptible to lead toxicity. Its impact is particularly severe and disproportionate in children because of their pattern of behaviour which makes them vulnerable to lead exposure [6]. It could damage the histology of the liver, affect the biochemical process as well as give rise to increase in the level of liver enzymes. Autopsy studies of lead exposed humans reveals that the largest storage of lead (33%) among the soft tissues is the liver followed by the cortex and medulla of the kidney [7].
The liver is the largest organ, accounting for approximately 2% to 3% of average body weight. The liver has 2 lobes typically described in two ways, by morphologic anatomy and by functional anatomy [8]. Located in the right upper quadrant of the abdominal cavity beneath the right hemidiaphragm, it is protected by the rib cage and maintains its position through peritoneal reflections, referred to as ligamentous attachments. Although, not true ligaments, these attachments are avascular and are in continuity with the Glisson capsule or the equivalent of the visceral peritoneum of the liver. The liver is one organ that is seen to be susceptible to heavy metal toxicity, including lead [9,10]. The liver is a vital organ with a wide range of functions such as detoxification, protein synthesis and production of biochemical necessary for digestion. It is actively involved in many metabolic functions and is the frequent target for a number of toxicants [11]. Hepatic damage is associated with distortion of metabolic functions which include detoxification, protein synthesis, and digestive functions [12]. Lead is a chemical element that has the symbol Pb from the Latin plumbum and with the atomic number of 82.In its pure form, it appears bright and silvery with a hint of blue [13] when it comes in contact with moist air, it becomes tarnished giving a dull gray colour. It is a weighty metal which is heavier than most of the common materials. It is a soft and malleable substance which has a relatively low melting point [14].
Lead is found at low levels in Earth’s crust, mainly as lead sulfide. However, the widespread occurrence of lead in the environment is largely the result of human activity, such as mining, smelting [15]. As lead is an element, once it is released into the environment, it persists. Because of lead’s persistence and potential for global atmospheric transport, atmospheric emissions affect even the most remote regions of the world [16]. Metallic lead beads dating back to 7000-6500 BCE have been found in Asia Minor and may represent the first example of metal smelting. At that time lead had few (if any) applications due to its softness and dull appearance. The major reason for the spread of lead production was its association with silver, which may be obtained by burning galena (a common lead mineral). The Ancient Egyptians were the first to use lead minerals in cosmetic, an application that spread to Ancient Greece and beyond. The Egyptians may have used lead for sinkers in fishing nets, glazes, glasses, enamels, and for ornaments. Various civilizations of the Fertile Crescent used lead as a writing material, as currency, and as a construction material. Lead was used in the Ancient Chinese royal court as a stimulant [17], as currency [18], and as a contraceptive; [19]. The Indus Valley civilization and the Mesoamericans used it for making amulets; and the eastern and southern African peoples used lead in wire drawing [20].
Lead tablets were commonly used as a material for letters. Lead coffins, cast in flat sand forms, with interchangeable motifs to suit the faith of the deceased were used in ancient Judea. Lead was used to make slings bullet from the 5th century BC. In Roman times, lead sling bullets were amply used, and were effective at a distance of between 100 and 150 meters. The Balearic slingers, used as mercenaries in Carthaginian and Roman armies, were famous for their shooting distance and accuracy. Lead was used for making water pipes in the Roman Empire; the Latin word for the metal, plumbum, is the origin of the English word "plumbing". Its ease of working and resistance to corrosion ensured its widespread use in other applications, including pharmaceuticals, roofing, currency, and warfare [21]. Writers of the time, such as Cato the Elder, Columella, and Pliny the Elder, recommended lead (or lead-coated) vessels for the preparation of sweeteners and preservatives added to wine and food. The lead conferred an agreeable taste due to the formation of "sugar of lead" (lead (II) acetate), whereas copper or bronze vessels could impart a bitter flavor through verdigris formation. “This metal was by far the most used material in classical antiquity, and it is appropriate to refer to the (Roman) Lead Age. Lead was to the Romans what plastic is to us. “Heinz Eschnauer and Markus Stoeppler" Wine-Anenological specimen bank” [22].
Human occupational exposure can also take place during the application and removal of protective lead-containing paints, during the grinding, welding and cutting of materials painted with lead-containing paints, such as in ship building, construction, demolition industries, and fabrication of heavy lead glass and crystal, and in crystal carving. Mining, smelting, and informal processing and recycling of electric and electronic waste can also be significant sources of exposure. The use of leaded petrol (gasoline) is another significant source of exposure [23,24]. Lead has no confirmed biological role, and there is no confirmed safe level ofl ead exposure. A 2009 Canadian- American study concluded that even at levels that are considered to pose little to no risk, lead may cause "adverse mental health outcomes". Lead is toxic for virtually all organs of the body and has significant debilitating effects on the nervous, renal, hepatic andh ematopoietic systems [23,25,26] Its prevalence in the human body-at an adult average of 120 mg[t]- is nevertheless exceeded only by zinc (2500 mg) and iron (4000 mg) among the heavy metals. Lead salts are very efficiently absorbed by the body. A small amount of lead (1%) is stored in bones; the rest is excreted in urine and faeces within a few weeks of exposure. Only about a third of lead is excreted by a child. Continual exposure may result in the bioaccumulation of lead. Because of its corrosion resistance, lead is used as a protective sheath for underwater cables [27, 28].
Materials and Methods
Adult wistar rats weighing 120-250 g at the time of first treatment were used. The animals were maintained on a 12- hour light /dark cycle with lights on at 8:00 A.M., with ad libitum access to food and water. The experimental animals were kept at an ambient temperature between 28-30oC throughout the period of acclimatization that spanned for two weeks. They were kept in cages in the animal house of Department of Human Anatomy, Ladoke Akintola University of Technology, Ogbomosho, Oyo State. The rats were maintained in accordance with the “Guide for the care and use of Laboratory Animals” prepared and compiled by the National Research Council [29].
The rats were fed with grower mash, had free access to distilled water and animals were weighed on a weekly basis.
Experimental design and grouping
The animals were selected based on their weights and were divided into four groups A, B, C and D with nine rats in each group. Group A rats were the controls, group B, C and D were experimental treated groups.
Group A consisted of nine wistar rats, which served as control group and they were fed with standard diet and water.
• Group B consisted of nine wistar rats which received 0.0 9 g/kg of lead and were fed with standard diet and water 28 days.
• Group C consisted of nine wistar rats which received 0.1 8 g/kg of lead acetate and were fed with standard diet and water for 28 days.
• Group D consisted of nine wistar rats which received 0.2 g/kg of lead acetate and were fed with standard diet and water for 28 days.
All groups were given a certain amount of feed and water including the control group. Lead acetate was administered orally through the oral cannula to the rats for 28 days after which they were sacrificed on the 29th day of experiment. The day after the 28th day of administration, that is, on the 29th day, the rats were sacrificed one after the other through cervical dislocation to make them to be in a near living state while still breathing. The whole liver was harvested and weighed using a sensitive weighing balance after which it was fixed in 10% normal saline and stored in sample bottle for tissue preparation, the bottle was labeled according to the group in which the animal belonged.
The tissue was subjected to chemical treatment in order to preserve the shape-structure relationship and chemical constituents of the liver tissue and cells. This was also done to prevent autolysis and putrefaction. The fixing fluid used was 10% normal saline and the liver was fixed in it. The Wistar rats were euthanized on 29th day of the treatment and the liver of each rat was immediately removed by dissection and fixed in 10% buffered formalin in accordance with previous investigators for light microscopy using Hand E as described by Wilson and Gamble.
Enzyme assay
Serum levels of Aspartate Transaminase (AST), Alanine Transaminase (ALT) and Alkaline Phosphatse (ALP) were determined using colorimetric methods (Haussament; Varleyet) using Randox Diagnostic kits. The assay of AST followed a modification of the colorimetric method reported by Varleyet. 0.1 m aliquot of haemolyzate was added to 0.5 m substrate mixture containing 100 mm phosphate buffer (pH 7.4), 2 mm 2- oxoglutarate and 100 mm L-Aspartate. Following incubation of the final mixture at 37oC for 30 minutes, the reaction was terminated by the addition of 0.5 ml of 1 mm 2,4- dinitrophenylhyrazine in 1 mm hydrochloric acid. After allowing the mixture to stand at room temperature for 20 minutes at 546 nm against a blank solution (no incubation at 37oC and termination solution added before addition of the haemolysate). Pyruvate solution of varied millimolar concentrations were used to prepare a standard curve from which AST activities were computed (Varleyet).
Alanine Transaminase (ALT) assay was carried out as described for AST except that 200 mm DL-alanine replaced L-aspartate in the procedure. The activities of AST and ALT were expressed in international unit/litre (IU/L). ALP activity determination followed the procedure reported by Haussament,using pnitrophenyl phosphate as substrate in 1m di-ethanolamine buffer (pH 9.8) containing 0.5 ml MgCl2.A0.02 ml of aliquot of haemolysate was added to 1 ml of buffered substrate solution and incubated as 30oC. The absorbance of the reaction mixture was read at 405 nm at 30 intervals for 3 minutes.
All data were presented as Mean ± SEM. Statistical analysis of the data in study in this study was carried out appropriately and tested for significance using the Student’s-t-test (t- distribution). P valuesË?0.05 were considered statistically significant. Statistical Programme for the Social Sciences (SPSS) software version 11.0 was used for the statistical analysis.
Statistical analysis
Experimental data that were obtained from this study were expressed as Mean ± SEM (standard error of mean) and data were analyzed by one way analysis of variance (ANOVA). Student’s t-test was employed to compare differences between the groups using Graphpad Prism for windows (GraphPad Prism, Inc Chicago). A value of p<0.5 was considered to be significant (Table 1).
| Groups | Weight(g) | Relative organ weight (%) |
|---|---|---|
| A | 4.955 ± 0.25 | 2.22 |
| B | 4.645 ± 0.33 | 2.06 |
| C | 6.422 ± 0.70* | 3.42 |
| D | 4.057 ± 0.24* | 2.67 |
Significance: p<0.05, value greater than 0.05 were considered insignificant while values less than 0.05 were considered significant (*). Values were expressed as mean ± Standard error of mean.
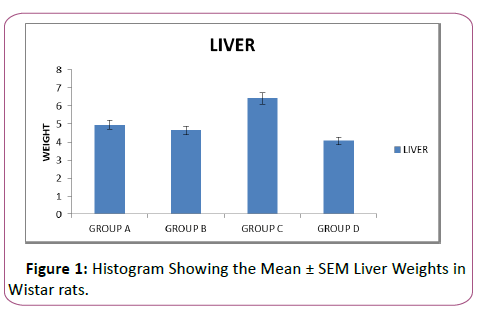
Table 1: Showing the mean ± sem of liver weight.
Results
The weights analysis for the liver showed an insignificant decrease (p>0.05) in weight, comparing control group to group B. Also there was a significant increase and decrease (p<0.05) in the weights of liver in group C and D respectively when they were compared with the control Group A in Figure 1. Group D which received the highest dose has the lowest liver weights compared to other lead-treated groups and group A, the control group
The graph showed the effect of lead acetate on the liver weights. The graph showed that the rats in group B have insignificantly decreased (p>0.05) liver weights compared to the liver weights of group A (control).There was a significant increase (p<0.05) in liver weights of the rats in group C compared to group A. Similarly, there was a significant decrease (p<0.05) in liver weights in the rats in group D when compared with rats in group A (Figure 1) and (Table 2).
| Groups | ALP | AST | ALT |
|---|---|---|---|
| A | 18.93 ± 0.9983 | 66.66 ± 3.42 | 33.00 ± 0.98 |
| B | 24.90 ± 1.55* | 95.10 ± 10.05* | 39.09 ± 1.05** |
| C | 32.63 ± 4.365* | 91.89 ± 4.64** | 41.69 ± 1.11*** |
| D | 35.44 ± 2.187*** | 109.0 ± 9.46** | 36.21 ± 0.18* |
Significance: p<0.05, value greater than 0.05 were considered insignificant while values less than 0.05 were considered significant (*). Values were expressed as mean ± Standard error of mean.
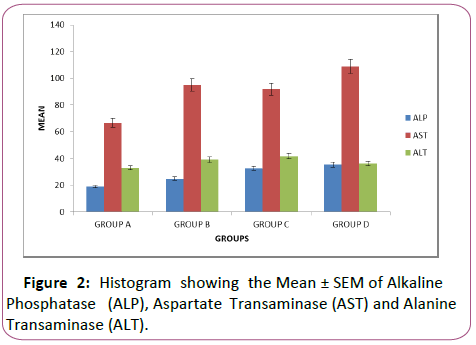
Table 2: Showing the mean ± sem of alkaline phosphatase (alp), aspartate transaminase (ast) and alanine transaminase (alt).
Biochemical evaluation
This table revealed significant increase (p<0.05) in the level of ALP in the lead-treated rats when compared with the control.
It increased from 18.93 ± 0.9983 to 24.90 ± 1.55 in group B, 32.63± 4.365 in group C and 35.44 ± 2.187 in group D.
Similarly, the level of AST increased significantly (p<0.05) in the treated groups compared with the control, it increased from 66.66 ± 3.42 in group A to 95.10 ± 10.05 in group B, to 91.89 ± 4.64 in group C and 109.0 ± 9.46 in group D There was also a significant increase (p<0.05) in the level of ALT in the leadtreated groups compared with the control, it increased from 33.00 ± 0.98 in group A to 39.09 ± 1.05 in group B, to 41.69 ± 1.11 in group C and 36.21 ± 0.18 in group D (Figure 2)
Graph revealed effect of lead acetate on the activities of ALT, ALP, AST in the liver in all treated groups B to D compared to the control group A. Histogram showed significant increase (p<0.05) in the activities of ALP, AST, ALT in all treated groups B to D when compared to the control (Figures 3A and 3B)
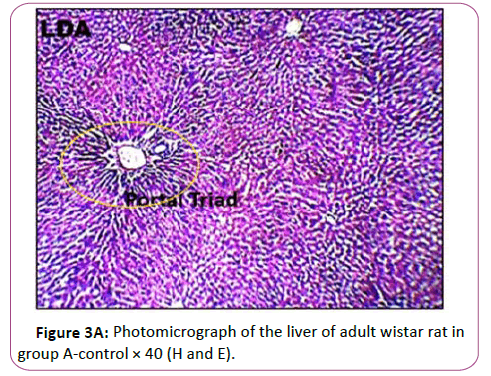
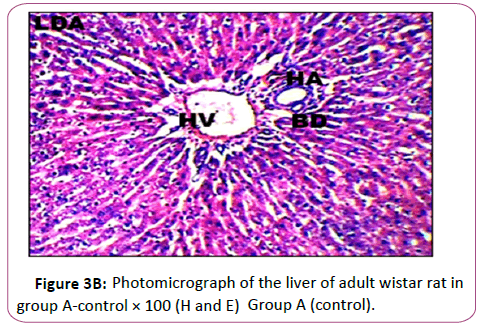
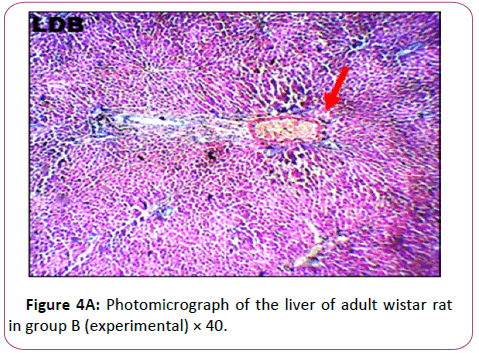
Figures 3A and 3B Photomicrograph of control group showing a normal histological feature of the liver. H and E staining shows the general cytoarchitecture of the panoramic view of liver general histo morphological presentations in Wistar rats in the control group. The portal triad (PT) consisting the hepatic vein (HV), hepatic artery (HA) and the bile duct (BD) as well as the distributed hepatocytes are demonstrated (Figures 4A and 4B).
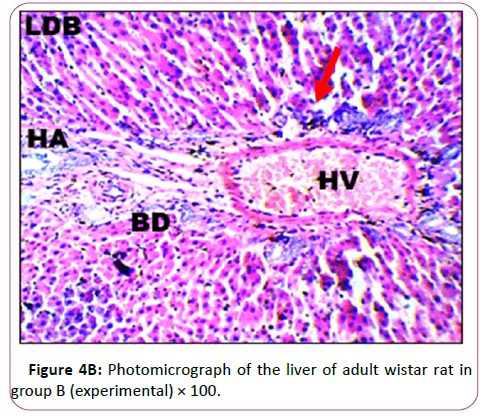
Figures 4A and 4B are the micrograph showed plate of hepatic cells in group B treated with 0.09 g/kg of lead acetate for 28 days. Group B: Exposed to 0.09 g/Kg of lead acetate.
It showed areas with moderately altered histomorphological features characterized by loss of liver parenchyma, disorganization of hepatocellular profile, cell death, dilation of the central vein, severe hemorrhage and presence of inflammatory red cells as well as fibrosis within and around the central vein and hepatic sinusoids are indicated with red arrows (Figures 5A and 5B).
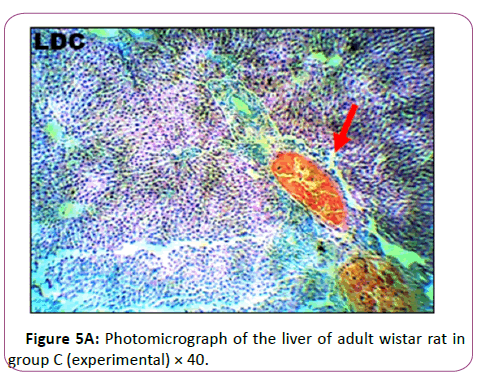
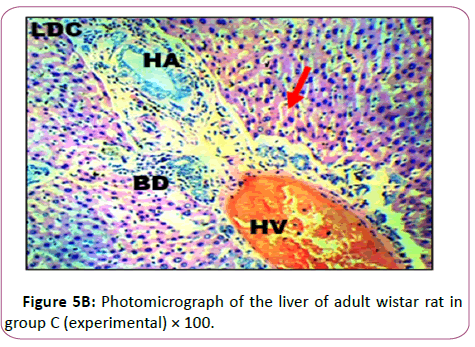
Group C: Exposed to 0.18 g/Kg of lead acetate in Figure 5B has The micr ograph showed plate of hepatic cells which sh ows in group C animals treated with 0.18 g/kg of lead acetate for 28 days. It showed areas with severely altered histomorphological features characterized by loss of liver parenchyma, disorganization of hepatocellular profile, cell death, dilation of the central vein, severe hemorrhage and presence of inflammatory red cells as well as fibrosis within and around the central vein and hepatic sinusoids are indicated with red arrows (Figures 6A and 6B ).
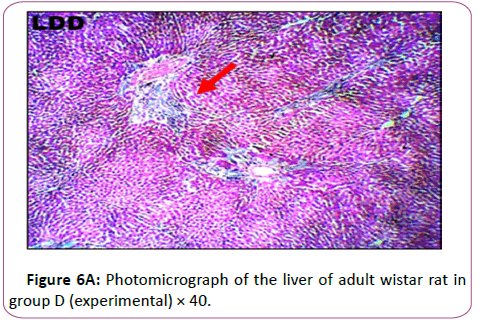
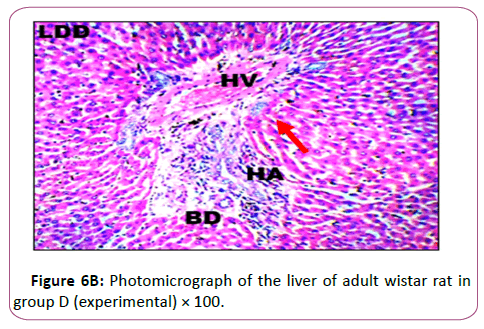
Figure 6B shows the Results from the micrograph showed plate of hepatic cells in group D administered 0.2 g/kg of lead acetate for 28 days. The plate showed areas with severely altered histomorphological features characterized by loss of liver parenchyma, disorganization hepatocellular profile, cell death, dilation of the central vein, severe hemorrhage and presence of inflammatory red cells as well as fibrosis within and around the central vein and hepatic sinusoids) indicated with red arrows
Discussion
This study was designed to observe some effects of lead on the liver of adult wistar rats and to find its effects on body weights of rats, mean and relative organ weights in the adult wistar rats. Comparing the first week of administration to the last week of administration, the bodyw eights of animals in group B which were exposed to 0.09 g/kg decreased insignificantly (p>0.05) when compared with the body weights of animals in group A. The body weights of animals in Group C which were exposed to 0.18 g/kg also decreased insignificantly (p>0.05) when compared to group A. The body weights of animals in the lead treated group D which received 0.2 mg/kg decreased significantly (p<0.05)c ompared ot group A. The reduction in body weights in treated rats is possibly attributed to lower food intake from the toxic effects of lead acetate. Previous reports has shown decreased body weights which was observed when 0.1% lead acetate was administered to male rats during 11 months [30]. Moreover, the results of the body weights were in accordance with other studies showing that exposure of Wistar rats to lead acetate causes a decrease in body weights [6,31-33]. The loss of body weights with continuous exposure to lead might also be explained by anorexia which is induced by heavy metal ingestion [34]. Another likely explanation for the loss of body weights may be the decreased muscle mass and cachexia due to oxidative stress induced by lead. Previous report has indicated that heavy metal toxicity is associated with oxidative stress [35,36]. Which according to many researchers is associated with muscle wasting and cachexia leading to low body weight [37,38].
were compared with the control group of non-exposed rats (group A). There was a significant increase (p<0.05) in liver weights of the rats in group C exposed to 0.18 g/kg of lead compared with the control group A while there was a significant decrease (p<0.05) in liver weights of the rats exposed to high dose of lead of 0.2 g/kg when compared to the control group. Additionally, it has been observed that decrease in absolute liver weights which was demonstrated in this study is not only a consequence of decreased food consumption, but also from direct toxicity of the lead acetate, perhaps by malabsorption of nutrients from toxic effects on the gastrointestinal tract or by inhibition of protein synthesis [39]. Furthermore, there was a significant increase (p<0.05) in the levels of ALT (alanine transaminase) in all the lead treated groups i.e. group B, C and D. It increased from 33.00 ± 0.98 in the control group to 39.09 ± 1.05 in animals in group B exposed to 0.09 g/kg of lead, to 41.69 ± 1.11 in the animals in group C rats which were treated with 0.18 g/kg of lead, to 36.21 ± 0.18 in the animals in group D which were treated with 0.2 g/kg of lead. A significant increase (p<0.05) in the level of AST (aspartate transaminase) in all the lead-treated groups (B, C and D) was also observed. It increased from 66.66 ± 3.42 in the control group to 95.10 ± 10.05 in group B, to 91.89 ± 4.64 in group C to 109.0 ± 9.46 in group D. The liver enzymes (AST and ALT) are considered as important biomarkers for the detection of lead hepatotoxicity. According to results from this study, lead caused a significant increase in AST and ALT levels compared to the control group. Increasing levels of AST and ALT in the plasma of treated rats is due mainly to the leakage of these enzymes from the liver cytosol into the blood stream [40]. Increase in the activities of ALT, AST and ALP in lead treated mice was shown to indicate liver dysfunction [41]. A significant increase (p<0.05) in alkaline phosphatase was observed in all the lead-treated groups (B,C and D) when compared to the control group. It increased from 18.93 ± 0.9983 in group A. (control group) to 24.90 ± 1.55 in group B, to 32.63 ± 4.365 in group C and to 35.44 ± 2.187 in group D. Elevation of alkaline phosphatase may indicate an injury to the biliary cells [42]. ALP is an enzyme that attaches to the cell membrane at varying affinity, influences the membrane permeability, and disrupts the metabolite transport. Also, the increased level of ALP has been repeatedly shown in animals that were exposed to lead. Besides, ALP has been shown to serve as a biomarker of cellular adjustment to the various injuring factors [43, 44].
Histological findings showed normal histoarchitecture in the control group A. The photomicrograph showed normal histological features .The portal triad (PT) comprising the hepatic vein (HV),hepatic artery (HA) and the bile duct (Bd) as well as the distributed hepatocytes are demonstrated. In the leadtreated groups (B,C and D).The photomicrographs showed areas with mild or severely altered histomorphological features characterized by loss of liver parenchyma, disorganization hepatocellular profile, cell death, dilation of the central vein, severe hemorrhage and presence of inflammatory red cells as well as fibrosis within and around the central vein and hepatic sinusoids were indicated with red arrows. Previous study has shown that liver histopathological examination in lead-treated rats revealed remarkable degenerative changes such as diffuse disorganization of hepatic cords vascular congestion, dilatation of sinusoids capillary, central vein and portal space. Mild lymphoid and mononuclear infiltrations were observed within the portal areas and central vein, the wall of which was dislocated. Nuclei chromatin was fragmented and cytoplasm contained many vacuoles. We observed a variation in the size of hepatocytes, some have deeply stained nuclei and homogeneous and acidophil cytoplasm [45]. This supports the conclusion made from previous research that lead is a very toxic agent which affects many vital organs, such as the liver [46].
Conclusion
This study concluded that Lead caused observable disruptions of the liver in the treated animals in form of histological disruption such as distortion of the liver parenchyma, cell death, disorganization of hepatocellular profile, dilation of central vein, severe hemorrhage, presence of inflammatory red cells and fibrosis within and around the central vein and hepatic sinusoids. Increase in the biochemical enzymes (ALP, AST and ALT) could also be indicative of liver dysfunction and an injury to the biliary cells. It can be concluded from this study that lead acetate is a strong hepatotoxic agent which has adversely affected the hepatic tissue of the investigated wistar rats.
References
- Lead Air Carl A.B, Edward E.A (2001) Saunders W.B. Quality Guidelines, 2 edn, WHO and Regional Office for (USA). World Health Organization (WHO) 497-520.
- Xinteras C (1992) Impact of lead contaminated soil on public health, science corner. Agency for Toxic Substance and Disease (ASDR) Registry, U.S. Department of Health and Human Services Atlanta, Georgia 1-27.
- Enuneku, A.A. (2010) Toxicological and biochemical alterations in adult amphibians (Hoplobatrachusoccipitalisand Bufo maculates) exposed to cadmium and lead. Ph.D Thesis, University of Benin,Nigeria.
- Haji A, Amir H (2012) The effect of occupational exposure to lead on blood hemoglobin concentration workers in Kermanshah oil refinery. IJT 6(19): 766-770.
- Onyeneke E.C, Omokaro E.U (2016) Effect of occupational exposure to lead on liver function parameters. Intl. J. Pharm. Med. Sci 6 (1): 15-19.
- Ibrahim NM, Eweis EA, El-Beltagi HS, Abdel-Mobdy YE (2012) Effect of lead acetate toxicity on experimental male albino rat. Asian Pac J Trop Biomed 2(1): 41-46.
- Mudipalli A (2007) Lead hepatotoxicity and potential health effects. Indian J Med Res 126(6): 518-527.
- Bismuth H (1982) Surgical anatomy and anatomical surgery of the liver. World J Surg 6(1): 3–9.
- Sivaprasad R, Nagaraj M, Varalakshmi P (2004) Combined Efficacies of Lipoic Acid and 2, 3-Dimercaptosuccinic Acid against Lead-Induced Lipid Peroxidation in Rat Liver. J Nutr Biochem 15(1): 18-23.
- Valko M, Morris H, Cronin M.T (2005) Metals, Toxicity and Oxidative Stress. Curr. Med. Chem. 12: 1161-1208.
- Meyer S.A, Kulkarni A.P Hodgson E, Smart R.C,Eds. (2001) Hepatotoxicity. Introduction to Biochemical Toxicology, 3rd Edition, John Wiley and Sons, New York, 487.
- Wolf P.L (1999) Biochemical Diagnosis of Liver Disease. Indian J Clin Biochem 14: 59-64.
- Greenwood N.N, Earnshaw A (1997) Chemistry of the Elements(2nd ed.). Butterworth-Heinemann.
- Thornton I, Rautiu R, Brush S. M (2001) Lead: The Facts (PDF). International Lead Association.
- IARC. Summaries & evaluations: Inorganic and organic lead compounds. Lyon, International Agency for Research on Cancer.(2006) IARC Monographs for the Evaluation of Carcinogenic Risks to Humans Vol.87.
- Hernberg S (2000) Lead Poisoning in a Historical Perspective. Am J of Ind Med 38(3):244-54.
- Winder C (1993b) "The history of lead —Part 3".LEAD Action News.2 (3). Archived from the original on 31 August 2007.Retrieved 12 February 2016.
- Yu L, Yu H (2004) Chinese Coins: Money in History and Society. Long River Press.
- Bisson M. S, Vogel J.O (2000) Rowman& Littlefield. Ancient African Metallurgy: The Sociocultural Context.
- Bisel S.C, Bisel J. F (2002) "Health and nutrition at Herculaneum". In Jashemski, W.F. Meyer F. G (eds.). Cambridge University Press. The Natural History of Pompeii. pp. 451–75.
- Eschnauer H. R, Stoeppler M (1992) "Wine An enological specimen bank". In Stoeppler, M.(ed.). Elsevier Science. Hazardous Materials in the Environment. 49–72(58).
- Patrick L (2006) Lead toxicity, a review of the literature. Part 1: Exposure, evaluation, and treatment. Altern Med Rev 11(1): 2–22.
- Xu J, Yan HC, Yang B, Tong LS, Zou YX et al. (2009) Effects of lead exposure on hippocampal metabotropic glutamate receptor subtype 3 and 7 in developmental rats. J Negat Results Biomed 8: 5.
- Landrigan PJ (2002) The Worldwide Problem of Lead in Petrol. Bulletin of the WHO 80(10).
- Siposet P, Szentmihalyi K, Feher E, Abaza M, Szilyagi M et al. (2003) Some effects of lead contamination on liver and gall bladder bile. Acta Biol Szeged 47(1-4): 139-42.
- Luckey T. D, Venugopal B (1979) Physiologic and Chemical Basis for Metal Toxicity. Plenum Press.
- "Toxic Substances Portal – Lead". (2011) Agency for Toxic Substances and Disease Registry.
- National Research Council, Neem: A Tree for Solving Global Problems(1992). National Academy Press, Washington, DC.
- Allouche L, Hamadouche M, Touabti A, Khennouf S (2011) Effect of long-term exposure to low or moderate lead concentrations on growth, lipid profile and liver function in albino rats. Advan. Biol. Res 5(6): 339-347.
- Shakoor A, Gupta PK, Kataria M, Dwivedi SK (2000) Effect of simultaneous exposure to aluminium and lead on growth in male albino rats. Indian J Toxicol 7: 51-56.
- AnnabiBerrahal A, Nehdi A, Hajjaji N, Gharbi N, El-Fazâa S (2007) Antioxidant enzymes activities and bilirubin level in adult rat treated with lead. C R Biol 330: 581-8.
- Xia D, Yu X, Liao S, Shao Q, Mou H, et al. (2010) Protective effect of Smilax glabra extract against lead-induced oxidative stress in rats. J Ethnopharmacol 130(2): 414-20.
- Klaassen CD (2001) Heavy metals and heavy-metal antagonists. In: Goodman and Gilman’s Pharmacological Basis of Therapeutics (10th edn.), McGraw-Hill, New York.
- Yeh YC, Liu TJ, Wang LC, Lee HW, Ting CT et al. (2009) A standardized extract of Ginkgo biloba suppresses doxorubicin-induced oxidative stress and p53-mediated mitochondrial apoptosis in rat testes. Br J Pharmacol 156(1): 48-61.
- Patra RC, Rautray AK, Swarup D (2011) Oxidative stress in lead and cadmium toxicity and its amelioration. Vet Med Int 2011: 457327.
- Kondo H, Nakagaki I, Sasaki S, Hori S, Itokawa Y (1993) Mechanism of oxidative stress in skeletal muscle atrophied by immobilization. Am J Physiol 265(6 pt 1): E839-44.
- Buck M, Chojkier M (1996) Muscle wasting and dedifferentiation induced by oxidative stress in a murine model of cachexia is prevented by inhibitors of nitric oxide synthesis and antioxidants. EMBO J 15(8): 1753-1765.
- Minnema DJ, Hammond PB (1994) Effect of lead exposure on patterns of food intake in weanling rats. Neurotoxicol Teratol 16(6): 623-629.
- Concepción Navarro M, PilarMontilla M, Martín A, Jiménez J, PilarUtrilla M (1993) Free radical scavenger and antihepatotoxic activity of Rosmarinustomentosus. Planta Med 59(4): 312-314.
- Alwaleedi, S.A (2015) Haemato-biochemical changes induced by lead intoxication in male and female albino mice. Int. J. Recent Sci. Res.6: 3999–4004.
- Fauci A, Braunwald E, Kasper D, Hauser S, Longo D (2008) Harrison's Principles of Internal Medicine, McGraw-Hill Professional, United States. Retrieved January, 25, 2010.
- Moussa SA, Bashandy SA (2008) Biophysical and biochemical changes in the blood of rats exposed to lead toxicity. Rom J Biophys 18(2): 123-33.
- Rafiei H, Ahmadi Z, Ashrafizadeh M (2018) Effects of Orally Administered Lead acetate II on Rat Femur Histology, Mineralization Properties and Expression of Osteocalcin Gene. IBBJ 4 (3).
- Haouas Z, Sallem A, Zidi I, Hichri H, Mzali I, et al. (2014) Hepatotoxic Effects of Lead Acetate in Rats: Histopathological and Cytotoxic Studies. J CytolHistol 5: 256.
- Sharma, A., Sharma, V. and Kansal, L. (2009) Therapeutic Effects of Allium sativumon Lead-Induced Biochemical Changes in Soft Tissues of Swiss Albino Mice. Pharmacognosy Magazine 5: 364-371.
- Saud A.A, Badr A.A, Doaa M.E, Mohamed A.D (2012) Effect of Corn Oil, Flaxseed Oil and Black Seed Oil on Lead Acetate-Induced Hepatic Tissue Damage: A Histological Study. J. Med. Plant Res 24(6): 4128-4134.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences